Rapid Listeria Detection Using the R.A.P.I.D.® LT Food Security System

In the United States, an estimated 2,500 persons become seriously ill with listeriosis each year and roughly 500 of those individuals die. Persons at increased risk include pregnant women (20 times more likely than other healthy adults to get listeriosis––one-third of all listeriosis cases occur during pregnancy), newborns, the elderly, persons who take glucocortico-steroid medications, those with weakened immune systems and persons with cancer, diabetes, kidney disease or AIDS.[1] Food producers need to protect themselves by ensuring that processed and prepared foods are not contaminated with Listeria. The use of a Listeria screening tool that is both rapid and accurate will permit earlier release of products without fear of potential outbreaks or possible food recalls. However, meeting both technical and budget requirements is a challenge for all food producers.
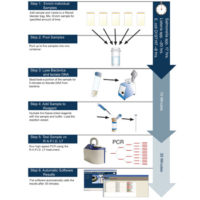
To enable easy, accurate and timely results, Idaho Technology developed the Listeria Food Security System (FSS), a PCR-based detection method that rapidly and specifically identifies Listeria species in or on food and environmental samples including Mexican-style soft cheese, turkey deli meat, stainless steel, ceramic tile and plastic. The system consists of the R.A.P.I.D. LT thermocycler and the AOAC-RI-approved Listeria LT test kit. Thermocycling is done in less than one hour and the entire test procedure takes 25–29 hours. The method involves a 24- to 28-hour single-step sample enrichment, mechanical lysis to release DNA, DNA amplification (PCR), internal amplification controls and an automatic result interpretation with a clear “positive” or “negative” call by the R.A.P.I.D. LT FSS software.
Easy to Use, Accurate and Timely
PCR is simple with this system because all PCR components are included in easy-to-use, freeze-dried reagent vials contained in the Listeria LT test kit. Freeze-dried reagents are stored at room temperature. The processed sample is used to rehydrate freeze-dried assay reagents with a buffer, and the mix is added directly to a PCR vessel. Adding to the ease of the system is sample enrichment in one step using nonproprietary media, along with simple setup and a true walk-away platform.
The test kit has two levels of specificity. PCR primers specifically amplify Listeria DNA, which is then detected by specific fluorescent probes that only detect Listeria. Thus, fluorescence detection occurs only if a Listeria target is amplified. The freeze-dried reagents also contain an internal amplification control to ensure that amplification is successful.
Additionally, the software incorporates a proprietary post-PCR melting procedure designed to increase the product’s sensitivity by using melting temperature and melting curves along with PCR amplification curves to determine the presence or absence of pathogens in a food or environmental sample. This additional feature provides users with increased confidence in the sample result. This kit is the first food-testing solution that provides both PCR and a melting-curve analysis combined with fast and reliable automated detection.
This system is as sensitive as the USDA and FDA methods at low inoculum levels in food and on environmental surfaces. Based on results of the validation study, this system was adopted as AOAC Performance-Tested MethodSM No. 010901 for the detection of Listeria in Mexican-style soft cheese and turkey deli meat and on stainless steel, ceramic tile and plastic.
The R.A.P.I.D. LT instrument is faster than traditional PCR instruments (< one hour versus three hours), because it uses air thermocycling to heat and cool the sample. Heating and cooling can be achieved quickly by varying fan speeds using a controlled heating coil, compared with other methods that require heating and cooling a metal block.
The sensitivity of PCR also allows for a shorter enrichment time than standard detection methods. One colony-forming unit (CFU) of Listeria in 25 g of food, or collected from a surface, can be detected after only 24–28 hours of enrichment using commercially available media at 30°C. DNA is then extracted from the Listeria bacteria by mechanical cell lysis. The bacteria are lysed directly after enrichment and diluted to remove PCR inhibitors before amplification.
Idaho Technology is committed to help save the world from deadly foodborne pathogens such as Listeria, Salmonella, E. coli O157:H7, L. monocytogenes and Campylobacter. Our goal is to make food safe and minimize pathogen outbreaks worldwide. The Listeria LT test kit promises to help food producers improve the quality and quantity of testing to protect their companies and their consumers from Listeria contamination.
www.idahotech.com
References
1. http://www.cdc.gov/nczved/dfbmd/ disease_listing/listeriosis_gi.html.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!