Acrylamide: Out of the Frying Pan and into the Fire (of Analytical Chaos)

If it wasn't for the innovative conscientiousness of those Swedish scientists last year, we'd all be munching on our fast food fries and dangerously delightful donuts in blissfully contented ignorance! Now, however, the consumer is being put in the uncomfortable position of "food chemist" regarding a naturally occurring compound that few can properly spell, let alone pronounce.
Those of you involved with chemistry and cooking know that there is no real difference: The reactions that food reagents undergo are plentiful and potentially drastic. Amino acids from proteinaceous materials can combine with saccharides from carbohydrate-containing foods and free fatty acids from triglyceride-based substances to create a wide array of new, and maybe not-so-improved, chemicals. This essentially is one of the more popular proposals in the current flood of acrylamide research that is out there for all to see and few to truly comprehend.
General testing to date shows the presence of acrylamide in various concentrations in most prepared foods, but mainly those containing proteins, carbohydrates and fat, which are exposed to heat. Unless we're rabbits (no offense intended to the vegans and vegetarians out there), that pretty much encompasses the "All-American" dietary intake. We'd be hard pressed to identify any commonly consumed food that has not been micro-waved, broiled, grilled, baked, roasted, braised, or woe upon us, fried! To a lesser extent, boiling and steaming also can affect the appearance of acrylamide.
Chemically, acrylamide is the primary amine of acrylic acid and is merely part of a variety of low molecular weight by-products created from the pyrolytic reactions of fats and oils with carbohydrate molecules. Acrolein produces the classic acrid, bitter smell when we burn butter in a frying pan. Acrylic acid--in addition to being the number one source of polyester-based plastics, with polyacrylamide gels the primary tool for biotechnology electrophoresis and water treatment--is found in small amounts in fermentation broth and from hydrogenation reactions of triglycerides.
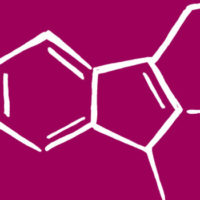
 From the molecular structures shown, it is easy to see the highly reactive nature of this molecule, and as current theory suggests, the presence of the amino acid arginine might cause the condensation reaction that produces the amide form of this three-carbon molecule (Figure 1). Again, to the dismay of the human consumer, arginine is present in so many food materials, both plant and animal, that it would be impossible to eliminate it from the food chain. Carbohydrate-rich foods, such as cereals, baked goods and breaded foods, are a mainstay of the human diet and cannot be eliminated from the food chain. Cooking foods is essential to both the quality and stability of the food, so this process cannot be eliminated from the food chain. What to do, what to do?
From the molecular structures shown, it is easy to see the highly reactive nature of this molecule, and as current theory suggests, the presence of the amino acid arginine might cause the condensation reaction that produces the amide form of this three-carbon molecule (Figure 1). Again, to the dismay of the human consumer, arginine is present in so many food materials, both plant and animal, that it would be impossible to eliminate it from the food chain. Carbohydrate-rich foods, such as cereals, baked goods and breaded foods, are a mainstay of the human diet and cannot be eliminated from the food chain. Cooking foods is essential to both the quality and stability of the food, so this process cannot be eliminated from the food chain. What to do, what to do?
Riding the Waves
This small and potentially elusive molecule is not the great analytical enigma that various private and government organizations dealing with the food industry are attesting. There are standard methods of analysis and commonly used analytical instruments and techniques available from the hallowed chambers of American Society for Testing and Materials (ASTM), the National Institute for Occupational Safety and Health (NIOSH), the U.S. Occupational Safety and Health Administration (OSHA), the U.S. Environmental Protection Agency (EPA) and other respected scientific groups. The acrylamide molecule is fairly easy to identify in many food matrices by a variety of analytical protocols with sufficient sensitivity to prepare a statistically accurate database of the relative distribution. A brief, yet thorough comparison of some of these previously published technologies is outlined below:
• EPA Method 8316 utilizes a simple isocratic high-performance liquid chromatography (HPLC) system, a commonly used C-18 column and water as the mobile phase at 195 nm ultraviolet (UV) detection. Sensitivity is estimated at 10 µg/L (parts per billion). A typical system costs approximately $12,000. Potential drawbacks are interferences at low-UV wavelengths. Improvements in accuracy can be made using higher resolution columns, by modifiying mobile phase with tetrahydrofuran (THF) and acetonitrile, and by increasing both the sensitivity and selectivity for acrylamide using a filter-based fluorimeter detector.
• EPA Method 8260 uses a gas chromatograph (GC) and a simple derivatization of the acrylamide to a volatile bromine-form with a clean separation on a capillary column and an electron capture detector (ECD). Sensitivity is estimated at 1 µg/L (ppb). A typical system costs approximately $12,000. Potential drawbacks include operator errors in the bromination treatment resulting in losses. Improvements in precision can be made by utilizing automated preparation techniques.
• ASTM has several guidelines for bulk acrylamide in air and water samples using simple UV spectrophotometric measurements. Sensitivity is approximately 500 µg/L (ppb), depending on the pathlength of the cuvette. Potential errors in data can arise from interfering substances, but this is a rapid technique for monitoring the levels of acrylamide in a food during a cooking process. Typical high-energy single-beam spectrophotometers cost app-roximately $5,500. Improvements can be made by using long-path cells to increase sensitivity and high-resolution optics to minimize interference peaks from other compounds.
• NIOSH, OSHA and several private companies that make field monitoring equipment for hazard assessment use highly specific colorimetric reactions, either on a solid substrate or solution, and employ "black-light" fluorescence tests for acrylamide and low-molecular weight polyacrylamide species. These have detection limits from approximately 500 µg/L (ppb) and higher, but take little time, cost little money and have relatively little error. With one-time-use test kits costing approximately $5 each, or filter fluorimeters costing less than $3,000, these should be considered viable options for measuring acrylamide in foods.
• Several international biotechnology firms are evaluating a series of promising enzymatic tests that can be applied to any food matrix, and provide rapid and virtually interference-free results in a short period of time. Cost per test is estimated at $5, and while the sensitivity is still under study, preliminary data shows it is less than 1 mg/L (ppm).
• The U.S. Food and Drug Administra-tion (FDA) released a very detailed procedure defined in a February 2003 draft document, "Detection and Quantitation of Acrylamide in Foods," which can be viewed at www.cfsan.fda.gov/~dms/acrypla2.html. The draft method described uses tandem liquid chromatography/ mass spectrometry/mass spectrometry (LC/MS/MS) and includes well-designed steps for the collection, pre-preparation, cleanup and introduction of the sample into the LC/MS system. Typical costs for a properly configured system range from $75,000 to $100,000. With the power of mass spectrometry, potential interferences are few, but several critical, operator-dependent steps involved in the procedure are prone to potential problems.
Considering Industry Applications
Based on concerns of regulatory agencies when it comes to dealing with the safety of the consumer, the FDA has opted to proceed with what is perceived by industry to be excessive caution and undue complexity in its approach to the acrylamide "issue." While the agency has presented its acrylamide method in draft form, many in the food industry who want to perform acrylamide testing believe that the tandem LC/MS/MS method proposed is the only method that can be used, because a very specific protocol with very specific manufacturers of the equipment are referenced. The general fear of the manufacturing community in risking potential or even perceived noncompliance is forcing many food manufacturers to exert much effort in either getting this exact equipment or seeking outside analytical laboratory support from those that have this equipment. Although the draft method is highly accurate and useful in many research and large-scale applications, some of the abovementioned alternative procedures should not be discounted as viable methods to track the appearance and content of acrylamide in a food manufacturer's process, especially for smaller sized processing operations.
For a proper understanding of what the industry needs to do to provide useful data to the regulatory agencies, whomever they may be, consider the following example that illustrates what industry does to food: A potato chip maker buys frying oil, potatoes and spice flavorings. The manufacturer prepares and treats the potatoes, fries them, flavors them and packages them. The manufacturer sells them and keeps masses of college students and the American economy moving forward!
If the potato chip maker's quality assurance laboratory has an analytical technique that can detect moderately low levels of acrylamide, then it is proper to extrapolate the ability of the lab to make a test for acrylamide at "time zero," before anything is processed, to establish a baseline level. During the multiple steps of the company's manufacturing process--preparation, treatment, frying, flavoring, and so on--any changes in this baseline can be monitored and logged. In the world of analytical techniques, this is comparable to the "method of additions," where a complex sample matrix prevents suitable calibration for accurate quantitative analysis. Any interferences will be compensated for, because the matrix of the food is a constant.
Using an HPLC or GC to monitor acrylamide in such a process application in order to generate statistically accurate data based on the trends in the formation of acrylamide should be acceptable to any regulatory agency. If the food industry can show that high-starch potatoes will yield lower levels of acrylamide than high-sugar potatoes, that's valuable data. If studies reveal a monounsaturated frying oil gives less acrylamide formation in a food product than a hydrogenated shortening, that's valuable data. If enough laboratories and small-sized food companies use such commonly available instruments to do independent tests on their manufacturing processes and variations in these processes (or treatments of the edible reagents in these processes), that's invaluable information.
Stay Informed to Quell the Storm
There is a number of ways food chemists can stay abreast of the developments in acrylamide detection and quanitification both for necessary research into this chemical as a potential health hazard in food and for applications in food manufacturing settings that are cost-effective, easy-to-use and accurate. Many references can be found online from a wide range of government, industry and scientific organizations. One of the most useful is the Joint Institute for Food Safety and Applied Nutrition's recently updated Acrylamide Infonet web page at www.acrylamide-food.org, which functions as a global resource and inventory of ongoing research on acrylamide in food.
Gerald J. DeMenna, Ph.D., is founder of Chem-Chek Laboratories, a food analysis lab whose clients include Herr's Potato Chips, Frito-Lay, Bonipak Foods and Green Acres. He also is the general manager and for Buck Scientific. A founding member of Food Safety Magazine's Editorial Advisory Board, DeMenna is also involved in industry organizations including the American Chemical Society and the Institute of Food Technologists. He can be reached at chemchek@aol.com.
Dhia Habboush, Ph.D., is Professor of Inorganic and Analytical Chemistry at Sacred Heart University, Fairfield, CT. He is the author of 30 research articles in the field of analytical chemistry.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!