Analytical Testing in Food Safety Continues to Grow

Many changes have occurred in the scientific and regulatory environment for food processors in recent years. Recognizing these changes were happening, we started the Food Safety Insights program here with Food Safety Magazine 4 years ago to report on these changes. Specifically, our stated goal was to report on what processors are doing in actual practice—not what people expect, think, or assume is happening. To accomplish this goal, we regularly conduct surveys and interviews with food processors, and to date, we have been in contact with thousands of processors around the world. Now that we are into our fourth year in reporting these facts to you, we wanted to highlight one of the key trends we have been seeing.
While we have found that a number of key changes have emerged, no trend has been mentioned more often or reported as being more impactful than the increase in the amount of analytical testing being conducted—especially the increase in testing for microorganisms. Processors have been making many changes to their sampling and testing plans, especially putting additional resources into microbiological and pathogen control programs.
The Growth in Microbiological Testing
In 2020, the global food microbiology market will conduct approximately 1.4 billion tests. Tests for indicator organisms, such as total viable organisms, coliforms, nonpathogenic Escherichia coli, and yeast/molds, are the most common tests, but roughly 350 million tests will be conducted for the market’s fastest growing category: the detection and identification of pathogens.
This growth has been driven by a greater focus on environmental monitoring (EM), with a majority of companies that we speak with reporting increases in the volume of EM tests that they collect. Companies tell us that they are increasing their volume of EM pathogen samples to find and eliminate resident pathogens in their plant. But even those processors who believe that they have control of their environmental pathogens and may not feel a need to search for these pathogens still report to us that they feel pressure from regulators [especially from U.S. Food and Drug Administration (FDA) “swab-a-thons”], customers, and auditors (many mention Safe Quality Food), all of whom are more frequently asking for more data regarding the performance of their EM programs.
More Comprehensive Environmental Monitoring
While many companies tell us that they have confidence in the performance of their EM program, nearly one-half have also told us that they have made changes to the sampling and testing that they do.
We spoke with many of these processors to get more background. They tell us that while they will be generally collecting more EM samples on a daily basis, they also report that a significant share of the increase in their sample volumes will be coming from “seek-and-destroy” sampling programs that they are now using frequently. They tell us that the increase in routine sampling plus their more focused hunt in difficult areas of their plant that routine sampling may have missed are allowing them to “…better clean their plant.” Many have described this as a switch in approach from a “shotgun to a rifle.” And this focus is clearly on EM sampling and testing as processors have not reported a commensurate increase in raw material or end-product testing.
Outsourcing to Eliminate Pathogens
This focus to find and get pathogenic harborage organisms out of the plant is also motivating more processors to get pathogens out of the plant laboratory. The need to enrich samples prior to analysis and to handle these high concentrations of pathogens in their in-plant lab, as well as the potential for those pathogens to find their way back to the production floor, are causes for concern. And processors are increasingly solving these issues by outsourcing their pathogen sample analysis to commercial food laboratories. This trend is having a dramatic impact on testing markets.
Outsourcing in manufacturing is typically used to reduce costs. But in this case, the motivation is almost always about food safety and removing a source of possible pathogen contamination, even if they find that outsourcing will be more costly than operating their own labs. In those instances involving larger processors where the economics of operating their own lab are highly favorable, many still elect to outsource their samples.
One of the processors we interviewed told us, “While we intend to maintain our in-house microbiology lab for the indicator organisms we test for, we are currently completing our bid process to eliminate our in-house pathogen testing. Once this process is complete, we expect that our overall costs will be 10 percent higher or more than our current costs, but we will have eliminated the need to work with pathogens in our plant and, we believe, reduced our risks.”
And this trend is relatively recent—with most of the shift occurring in the past 5 years or so. When we asked processors how long they have been outsourcing their pathogen samples, their most common answer was that they have only changed their testing processes and started outsourcing their pathogen samples within the past 4–5 years.
This decision—as with all decisions in business—is not immune from economics. This decision is easier for smaller and midsized companies, as it is difficult to justify the capital expense for a laboratory to analyze a small number of samples. In previous surveys, we have reported that approximately two-thirds of processors (by location) will outsource their pathogen analysis, whereas only about one-third (mostly the larger facilities) will continue to use their in-house lab. But because the larger processors are the ones with more samples, outsourced pathogen analysis by number of samples (rather than processor locations) is approximately 50 percent as measured by total pathogen sample volume.
Companies that have higher numbers of samples certainly have a better economic justification for investing in a lab, but we are more frequently seeing these larger facilities increasingly looking to send their samples out.
These processors are telling us that they are considering not only the cost of the daily operation of the lab but the additional costs of training, hiring analysts, certifications, and the added demand from customers and auditors for data from independent, third-party labs. Add all this together plus the benefit of getting pathogens out of the plant, and processors tell us that outsourcing becomes a far-less expensive and easier-to-justify decision.
Growth in the Commercial Lab Market
These factors are driving growth in the commercial food safety lab market. As processors elect to outsource more pathogen samples, commercial microbiology labs are seeing a high level of growth in their microbiology business. While we estimate that the overall volume in the microbiology testing business is growing approximately 5 percent, and pathogen testing volume growing approximately 6–7 percent, outsourcing is driving the volume of tests being sent to commercial labs by as much as 10 percent. Evidence of this is clear, and those in the commercial lab and diagnostics markets are certainly aware of this trend, including some very large outsourcing contracts that have been let recently by a number of large food processors.
This shift in sample analysis is potentially creating an opportunity for both laboratories and food processors. Laboratories are not only hoping that they can demonstrate that the basis of their relationship with processors should not be just about analyzing samples and price, but they also hope to demonstrate their overall value to food processors. If the laboratory companies are successful, this shift will not just be about the outsourcing of sample analysis but will also include processors working more cooperatively with their lab partners and using the laboratory’s expertise to optimize all areas of their program, including better selection of sample locations, numbers, and types, and the overall optimization of their program and costs. Evidence of this trend is also clear, as laboratories are seeing even larger growth rates in their training and service offerings.
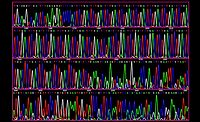
Whole-Genome Sequencing
While new technologies and scientific advances are changing food safety, few of these advances have been more impactful than whole-genome sequencing (WGS). Much of the impact from WGS has come from its use in traceback analysis and the potential to match the cause of an outbreak to its source. This impact has been amplified by FDA’s practice of collecting swab samples during inspections and submitting them for WGS analyses. This has led some to hypothesize that processors will want to collect their own samples for WGS analyses either as a defense against these efforts by FDA or as a way to find and rid their plants of harborage pathogens before FDA can find them. While we have seen a clearly increasing trend to employ seek-and-destroy projects to rid plants of pathogens, the use of WGS does not seem to be growing. Over the past 3 years, when asked about WGS use, no more than 7 percent of the processors that we spoke with in any survey—and typically fewer than that—said that they will be submitting their samples for WGS. We will continue to ask this question in our surveys on an annual basis and report when and if we see a change in this trend.
And as with WGS and all of these other topics, we will continue to study, test, and determine what is happening in food processing and bring you the most accurate market and reporting on trends as we have been doing since 2017. If you have something you would like us to find out, send us an email and let us know. We are always looking for new food safety insights.
Bob Ferguson is president of Strategic Consulting Inc. and can be reached at foodsafetyinsights@gmail.com or on Twitter at @SCI_Ferguson.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!