Food Safety
Antimicrobials for clean labels
Like it or not, today’s consumers want safe foods without complicated, unrecognizable chemicals and preservatives to ward off bacteria, mold and spoilage.

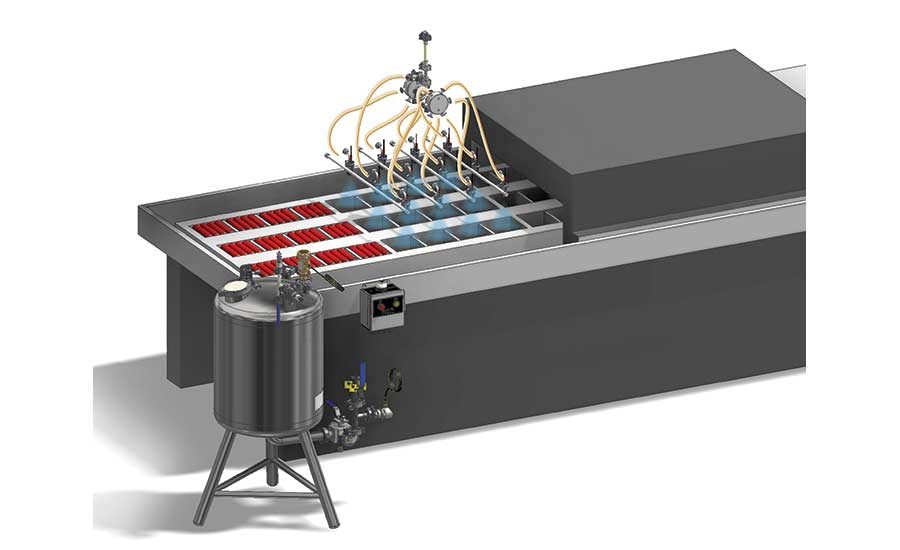
The SLIC® (Sprayed Lethality in Container) process, established by the USDA/ARS, improved the safety of RTE processed meat products such as hot dogs against food borne contaminants. The dose of the antimicrobial, lauric arginate, is dispensed by spray nozzles before or as the product is inserted into vacuum packaging prior to sealing. The system controller ensures the proper volume of antimicrobial is applied when operating conditions change. Source: Spraying Systems Co.


Butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA). They’ve been around for a long time and are still in use today as preservatives. Use either of them, and some consumers may shun your products.
We all want safe food, free of harmful bacteria, and we want long shelf life. But some of the chemicals used today as antimicrobials and preservatives have unpronounceable and unrecognizable names with possibly unknown consequences after consumption. Today, consumers want clean labels with preservatives and antimicrobials that they can recognize and understand: like vinegar, ascorbic acid, rosemary—or chemicals derived from organic or natural substances.
This makes it more complicated than ever to produce a product with a clean label that will appeal to picky consumers. Fortunately, some treatments have proven safe enough that they don’t have to be listed on the label, for example, chlorine, hydrogen peroxide, ozone or bacteriophages. But not to fear, there are some clean-label alternatives available for primarily antimicrobial usage and also for extending shelf life.
Article Index:
- Clean labels, natural and organic
- Antimicrobials derived from natural sources
- Mix them for greater benefit
- Chlorine, ozone, hydrogen peroxide, peroxyacetic acid
- Silver: Particles or ionic?
- Bacteriophages
Clean labels, natural and organic
The term “clean label” seems to mean slightly different things to different people. Clean label is a little like “all-natural,” and this is a problem when creating “clean” antimicrobials. You can start out with something organic and finish with something not so much. “Organic,” thanks to USDA, is better understood and defined.
USDA’s National Organic Program’s (NOP) List of Allowed and Prohibited Substances names four primary types of chemicals as being suitable for use as no-rinse sanitizers in fresh produce operations. These include, in short, sodium chlorite solution (acidified with citric acid), chlorine materials, ozone and peroxyacetic acid, according to Elis Owens, director, technical services, Birko in “FAQs on Organic Produce,” published on Birko’s website.
While the term “organic” has a specific meaning as defined by USDA, the terms clean label and natural tend to be less specifically defined—as we just noted. Delavau Food Partners offers a clean-label antimicrobial, which also can be used as a clean-label preservative.
“It appears on the label simply as fermented wheat flour and fermented starch, but offers performance comparable to traditional calcium propionate,” says Lawrence Marks, research and development manager for Delavau.
“Our clean-label antimicrobial products can be formulated to function in baked goods as a clean-label replacement for calcium propionate,” adds Delavau’s Matt Patrick, director, research development. “It inhibits mold growth, increasing the shelf life and sensory quality of the product. In many cases, the label reads simply: cultured wheat starch, wheat flour.”
Several plant and animal sources either possess antimicrobial properties when used directly in food or when extracts and enzymes are processed from these sources. Common spices and herbs known to have antimicrobial properties include the oils of cinnamon, cloves, basil, rosemary, oregano and garlic—to name a few. Animal sources include, for example, enzymes, such as lysozyme and lactoferrin; bacteriocins from microbial sources, such as natamycin and nisin; organic acids (e.g., citric and sorbic); and naturally occurring polymers, such as chitosan, which is derived from shrimp.
One ingredient processor, Primex of Iceland, submitted a 150-page application to FDA in August 2012 seeking GRAS approval of its ChitoClear product, which is derived from shrimp. ChitoClear is intended as an additive to designated foods, including meat and poultry, to be used as an antimicrobial agent, emulsifier, nutrient supplement, processing aid and stabilizer or thickener. The GRAS notice was later dropped at the request of the processor and ChitoClear appears not to be marketing to the food and beverage industry.
But are natamycin, nisin and chitosan clean label?
“These ingredients can be made from organic-certified raw materials and reagents, but that does not mean they will suit consumers’ clean-label and natural preferences,” says Delavau’s Marks. “The term ‘All Natural’ is not well defined or particularly valuable; moreover, these preservatives do not look very natural, and they do not sound healthy. They are mostly used in the bakery industry in a liquid form that is spray-applied to the surface of the baked product prior to bagging. Accordingly, they require spray atomization or other topical application equipment to be installed and maintained on the bakery line.”
Nisin-, chitosan- and natamycin-based products are not considered natural or organic, but they can be very effective for preserving cooked and fresh meats.
“Vinegar and cultured sugar products are some of the most effective label-friendly antimicrobial agents on the market today,” says Tom Rourke, director business development, Corbion. “And they are the ingredients used in our Verdad family of antimicrobial solutions. These products can often be combined with various spice blends to optimize flavor and suppress the growth of pathogens in meat and poultry products.”
Antimicrobials derived from natural sources
Large antimicrobial suppliers, like DuPont Nutrition & Health, offer several antimicrobial options, which can be combined with other solutions. Joseph Stanley, group manager - food protection, North America, breaks the company’s antimicrobial offerings into four categories: fermentates (MicroGARD), protective cultures (HOLDBAC), purified antimicrobials (Nisaplin and Natamax) and antimicrobial blends (BioVia and NovaGARD).
“Throughout history, beneficial microorganisms have been used to safely and naturally extend the shelf life of fermented foods [e.g., cheese salami and sauerkraut],” says Stanley.
The microorganisms used in modern day food fermentations preserve food mainly through product acidification, but also by other means, such as competitive exclusion, and through the production of metabolites (e.g., bacteriocins, enzymes, organic acids and other molecules), which inhibit the growth of spoilage organisms or pathogens.
Whole fraction fermentates (e.g., MicroGARD) production is similar to many traditional food fermentations, adds Stanley. Microorganisms with a long history of use, which are generally recognized as safe (GRAS), are selected based on the metabolites produced during the fermentation process and the spoilage or pathogenic organism(s) that need to be controlled. For example, propionibacteria strains, commonly used in cheese production, are known to produce high levels of propionic and acetic acid during fermentation. These organic acids have been shown to control certain Gram-negative bacteria and a variety of yeast and molds.
Other lactic acid bacteria (LAB) naturally produce metabolites that inhibit a variety of Gram-positive organisms, says Stanley. The selected organism(s) can then be used in controlled fermentations using various carbohydrate sources as the nutrient base (i.e., dextrose, non-fat dry milk, etc.) to produce desired antimicrobial metabolites.
Protective cultures (e.g., DuPont’s HOLDBAC) are used to extend the shelf life of fresh fermented dairy products, fermented meats and other fermented foods. These cultures are commonly used to protect yogurt, sour cream and fresh cheese, says Stanley. The protective cultures are generally added at the same time as starter cultures when manufacturing fermented foods.
During fermentation, the protective cultures grow alongside the starter cultures and produce a variety of antimicrobial metabolites, which help extend the shelf life of fresh fermented dairy products. In addition to metabolite generation, the protective cultures compete with undesired microorganisms for nutrients and extend product shelf life through competitive exclusion.
Another source for antimicrobials is through the fermentation and purification of microorganisms, such as Streptomyces natalensis, which is the method used to produce DuPont’s Natamax. The natamycin resulting from this fermentation process is concentrated, crystallized and dried before being standardized with lactose, salt or glucose. Natamycin is a potent antimycotic (antifungal) agent that is commonly used to protect cheese, baked goods and some salad dressings from yeast and spoilage. Compared to chemical preservatives, natamycin is active at very low concentrations (one to 40 ppm) against yeast and molds, says Stanley.

Nisin is another natural antimicrobial that’s been used worldwide since 1953 to control Gram-positive spoilage in heat-processed and low pH foods. Available as DuPont’s Nisaplin, the antimicrobial is active against a wide range of Gram-positive bacteria and ineffective against Gram-negative bacteria, yeasts and molds, adds Stanley. This nisin-based product is currently being used in a variety of applications, which include pasteurized processed cheese spreads, soups, sauces, salad dressings, liquid egg products and more.
A&B Ingredients focuses on lauric arginate as an antimicrobial that may be used in many ways, according to Gil Bakal, managing director. Lauric arginate is synthesized from lauric acid, a natural fatty acid found in coconuts, and arginine, an essential amino acid.
“Our lauric arginate product is sold under the trade name, CytoGuard LA,” says Bakal. “It is a powerful antimicrobial that is highly effective against a wide range of bacteria, including Listeria, Salmonella, E. coli, Campylobacter, yeast, molds and lactobacillus. In many applications, lauric arginate can be used as a processing aid.”
Lauric arginate is typically exempt from labeling as a processing aid, adds Bakal. One example is the sprayed-lethality-in-container (SLIC) method of application. SLIC is a patent-pending process designed to improve the safety of RTE processed meat products against foodborne contaminants. It is based on introducing an antimicrobial purge into the vacuum packaging of the RTE meat products before, or as the product is inserted, using the force of the vacuum to spread the antimicrobial purge around the product.
As an alternative to bathing, dipping or spraying, SLIC treats the surface of the meat product using minimal concentrations of antimicrobial and providing an extended duration of contact time. Lauric arginate usage is typically less than 50 ppm in these applications. Finally, the antimicrobial can also be used to treat incoming raw materials to reduce the risk of contamination, or it can be used in products as a first step to lower counts on high-risk items, like vegetables and meat products.
Mix them for greater benefit
“Many applications benefit from the use of more than one antimicrobial,” says John Wyatt, product manager - food protection, North America, DuPont Nutrition & Health. “For example, there may need to be protection from spoilage bacterial growth [from lactic acid bacteria] and from mold growth.”
The antimicrobials used to control spoilage bacteria are often different from those used to delay or prevent mold growth, adds Wyatt. DuPont has several blends that combine the antimicrobial capabilities of different products to provide a wider spectrum of protection than could be achieved by a single antimicrobial product.
“By mixing together multiple organic acids, such as L-lactic and acetic acids, we can safely and naturally add more ‘hurdles’ to inhibit the growth of spoilage and pathogenic bacteria in meat, poultry and other processed meats,” says Corbion’s Rourke.
Generally, a blend of organic acids leads to better shelf life and enhanced safety, which, in turn, ensure less food waste.
“Antimicrobials should be looked at for their mechanistic function,” says A&B Ingredients’ Bakal.
Antimicrobials with different mechanistic functions, when combined, often have an additive or synergistic effect. As an example, lauric arginate has the ability to damage and/or perforate cell membranes. When using lauric arginate with high-pressure pasteurization, studies have shown that less pressure and/or less time can result in the same or greater benefits. Also, when lauric arginate is used before organic acids, the antimicrobial effect of the organic acids becomes more pronounced, adds Bakal.
Chlorine, ozone, hydrogen peroxide, peroxyacetic acid
What do the above four antimicrobial chemicals have in common? The four antimicrobial chemicals do not have to be listed on the label by USDA facilities when they come into contact (e.g., sprayed on) with meat products. Typically, hydrogen peroxide is combined with acetic acid in peroxyacetic acid, hence the prefix, peroxy. In addition, all four can be used as washes/rinses for the sanitation of fruit and produce under USDA’s NOP.
Hydrogen peroxide, listed GRAS by FDA in 1986, is a powerful oxidant and antimicrobial that can also be used to sterilize aseptic packaging containers to prolong shelf life, clean and purify food products of unwanted substances (e.g., sulfur dioxide and residual chlorine) and bleach foods to improve color. A major advantage to using hydrogen peroxide is that there is no residue left as it breaks down to water and oxygen.
Ozone has been approved as an additive by FDA and can reduce levels of harmful microorganisms, such as E. coli and Cryptosporidium, in ingredient water supplies and juices, e.g., apple juice. However, under the requirements of the juice HACCP regulation, any treatment, including ozone, must be carried out as a process that will provide at a minimum a five-log reduction of the microorganism that is most likely to cause a health concern related to that product, for example, apple juice.
Under this regulation, a juice processor is advised to provide validation that the ozone process (or UV light or heat pasteurization) does indeed create a five-log reduction in the pertinent microorganism. Ozone is reported to have 1.5 times the oxidizing potential of chlorine and 3,000 times that of hypochlorous acid. Contact time for antimicrobial action with ozone is typically 1/4 to 1/5 the time needed for chorine solutions.
Chlorine/chlorine dioxide can be used in cereal flours and is also used as an antimicrobial agent in poultry process water at a concentration not to exceed three ppm residual chlorine dioxide. Used as an antimicrobial agent in water to wash fruits and vegetables (not raw commodities), residual chlorine level should not exceed three ppm.
Silver: Particles or ionic?
Currently, FDA allows the use of silver nanoparticles in antibacterial wound dressings, but it does not list any silver nanoparticle products in its Food Additive Status List. However, silver nanoparticles have been applied to food packaging materials, but there have been several studies concerning the migration of silver particles into the food contained in the package and whether this migration of silver to the food or beverage portends any health issues.
While the doctor is out on the safety issues concerning the ingesting of silver nanoparticles or colloidal silver, ionic silver is on the FDA list as silver nitrate, and an antimicrobial agent, in aqueous solution with hydrogen peroxide in bottled water treatment applications.
Another ionic silver compound has been applied to food contact surfaces.
“Silver dihydrogen citrate [SDC] is our proprietary ionic silver technology,” says Hank Lambert, CEO of PURE Bioscience, Inc. “SDC is the active ingredient in our hard surface disinfectant and food contact surface sanitizer, PURE Hard Surface, and in PURE Control, an antimicrobial food processing aid.”
PURE Hard Surface can be used in processing plants to disinfect and sanitize surfaces and the environment throughout the facility. The active ingredient falls under 40 CFR 180.940 and does not have to be rinsed from food contact surfaces when used as directed. The antimicrobial provides broad spectrum efficacy against the most common pathogens, including Salmonella, Listeria, E. coli, Campylobacter and norovirus, with the lowest EPA toxicity rating.
PURE Control is a food contact substance allowed by FDA and USDA for use on produce and poultry, and does not have any food labeling requirements, says Lambert. This non-toxic direct food contact intervention dramatically reduces pathogens without negative impact to organoleptics, product yield, the environment or equipment.
Bacteriophages
In August 2006, FDA approved the use of a bacteriophage preparation made from six individually approved phages as an additive on RTE meat and poultry products as an antimicrobial agent against Listeria monocytogenes (Lm). The approved phage preparation is reported to be effective against 170 strains of Lm. The 2006 document notes that use of this product comes under the Federal Meat Inspection Act or the Poultry Products Inspection Act, both administered by USDA. Labeling instructions are provided within this document, however, when phage preparations are applied to slaughtering/processing of raw carcasses, in most cases, there are no labeling requirements.
Most bacteriophage preparations have been approved for clean-label processing in the USA, EU, Canada, Australia, New Zealand, Switzerland and Israel, according to Micreos’ PhageGuard website. In the USA, FDA has granted GRAS for bacteriophages. Bacteriophages lyse and consume their host bacteria, and when finished, the host bacteria are depleted in the food. While bacteriophages have no other effect on food—they don’t change the flavor, texture or color—because of their specificity, they do not eliminate typical food spoilage bacteria.
Do phages work? A medium-sized smoked salmon producer on the East Coast had this to say about using an Lm-specific bacteriophage:
“We had a recall a few years back, and it cost us in excess of well over $100,000. The FDA came in, consultants came in, we lost business. We met Micreos [PhageGuard] at a seafood show shortly after, they came over [from Holland], and we started using Listex. That was almost 10 years ago.
“The problem with cold smoked salmon is that it is naturally contaminated and cannot receive a lethality treatment, such as heat, without inadvertently altering the typical characteristics of the product. Listex only targets Listeria, leaving everything else the way it is supposed to be. It’s the only product I have come across that does this for us. I sleep better knowing that I am controlling Listeria and not flying blind.”
Bacteriophages, in many cases, are certified organic, such as Listex. They also may be certified halal and kosher, but that should be verified with the individual supplier.
Bacteriophage technology has been advancing to include additional bacteria fighters in food safety. Intralytix Inc. recently announced that its ShigaShield has received GRAS approval from FDA. ShigaShield is aimed at controlling the foodborne/waterborne bacterial pathogen, Shigella, including three species, S. flexneri, S. sonnei and S. dysenteriae.
“Shigella spp. are one of the leading bacterial causes of diarrhea worldwide, causing an estimated 80 to165 million cases each year,” says Dr. Alexander Sulakvelidze, Intralytix’s chief scientist. “In the United States, Shigella are the third-most common causes of gastroenteritis, with more than 500,000 cases occurring annually. Another important potential application is to use ShigaShield for improving the safety of foods for the US military and for travelers, for example, for treating fresh fruits and vegetables in high-risk Shigella locations overseas where the local sanitation standards and/or quality of water are not optimal, thus creating an increased risk of Shigella contamination.”
For more information:
Joseph Stanley, DuPont Nutrition & Health, 800-255-6837,
joseph.stanley@dupont.com, www.danisco.com
John Wyatt, DuPont Nutrition & Health, 800-255-6837,
john.wyatt@dupont.com, www.danisco.com
Tom Rourke, Corbion, 800-669-4092,
foodus@corbion.com, www.corbion.com
Lawrence Marks, Delavau Food Group, 732-253-7779,
lmarks@delavau.com, www.delavaufood.com
Matt Patrick, Delavau Food Group, 732-253-7779,
mpatrick@delavau.com, www.delavaufood.com
Elis Owens, Birko, 303-289-1090,
eowens@birkocorp.com, www.birkocorp.com
Gil Bakal, A&B Ingredients, 973-227-1390,
gbakal@abingredients.com, www.abingredients.com
Hank Lambert, PURE Bioscience, Inc., 619-596-8600,
hlambert@purebio.com, www.purebio.com
Alexander Sulakvelidze, Intralytix, 410-625-2533,
asulakvelidze@intralytix.com, www.intralytix.com
Dirk de Meester, Micreos Food Safety, +31 317 421 414,
d.demeester@micreos.com, www.phageguard.com
This article was originally posted on www.foodengineeringmag.com.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!









