Home » regulations
Articles Tagged with ''regulations''
Regulatory Watch
Shared regulatory approach for livestock, poultry cell-cultured products
May 22, 2019
Food Safety
Cleaning, sanitizing programs are part of the food safety equation
March 20, 2019
Regulatory Watch
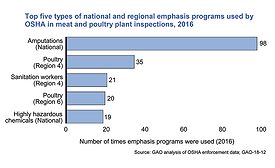
Final rule amends national list of allowed, prohibited substances
March 20, 2019
Never miss the latest news and trends driving the food safety industry
eNewsletter | Website | eMagazine
JOIN TODAY!Copyright ©2025. All Rights Reserved BNP Media.
Design, CMS, Hosting & Web Development :: ePublishing