Methods
Rapid Testing Methods—The Future
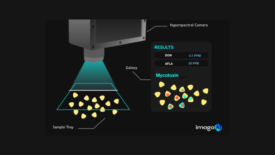
Most companies no longer have a microbiology lab or pathogen analysis capabilities, which will change the types of rapid test methods that will be in demand in the future
April 12, 2024
Never miss the latest news and trends driving the food safety industry
eNewsletter | Website | eMagazine
JOIN TODAY!Copyright ©2025. All Rights Reserved BNP Media.
Design, CMS, Hosting & Web Development :: ePublishing