Widening Recalls and Class Action Lawsuits: Alarming Recall Trends in 2024
The world of recall exposure and management is changing, making it more important than ever that you are fully protecting your company and brand

Video credit: SVTeam/Creatas Video+/Getty Images Plus via Getty Images
Food product recalls are funny things. Recalls are technically supposed to be "voluntary," but, in practical reality, they never truly are. Recalls should be as narrow in scope as reasonably possible; increasingly, however, regulators are demanding broader and more expansive recalls than ever before. Furthermore, recalls were never supposed to result in class action lawsuits being filed against the companies recalling the products—but now, they almost certainly do.
Each year, there are, on average, 614 recalls of food and beverage products. I know this because, as a food industry lawyer, I have been intimately tracking and analyzing food product recalls for decades. Nearly ten years ago, to facilitate these efforts and to give the food industry free access to important recall data, my firm began developing the Food Recall Reporter ("FRR").1 Today, FRR is the only open-access, searchable database in the world that contains detailed information about every Class I, Class II, and Class III recall initiated in the U.S. since the year 2000. The FRR has been used extensively by industry, trade associations, regulators, academia, and media to research and explore decades of recall data, trends, and precedence.
Over the years, the FRR has allowed us to observe important recall data and advise our clients on the latest scientific and regulatory developments in the execution and management of recalls. In this article, we will share our perspective on some recall-related "hot topics" from 2024, take a close look at the main drivers of recalls in 2024 (and previous years), and offer predictions on what may be in store for 2025.
What Drove Recalls in 2024?
So, what did we learn from 2024? As noted above, while recalls are technically supposed to be a "voluntary" exercise, in practice, they rarely ever are. As a general matter, the U.S. Department of Agriculture (USDA) and U.S. Food and Drug Administration (FDA) manage the recall process somewhat differently.
USDA likes to adopt a more "hands-on" approach than FDA. When USDA learns about an issue with a product, the agency summons its recall committee, which usually comprises more than two dozen regulators. The full USDA Recall Committee then corners the company leadership on a telephone conference call to demand that the company agree to a recall, which, in many cases, USDA has already defined. The USDA Recall Committee also authors its own USDA "Recall Notice," and then grants the company it is targeting roughly 30 minutes to review and "approve" the Recall Notice. Even if approval is not given, the agency will publish the Recall Notice the agency drafted, for public consumption, at the expiration of the 30 minutes. There really is nothing about this process that is voluntary.
When dealing with USDA in this context, it is important to force the agency:
- To provide critical and material information to aid the company's recall decision-making process.
- To answer questions about the agency's current thinking about the need for, and scope of, any potential recall.
- Where appropriate, to say "no." This is because, sometimes, the agency's thinking is wrong, and the decision to initiate any recall (or even agree to any requested scope) would be unreasonable.
On the FDA side, the agency does take a slightly more "hands-off" approach. Usually, most issues relating to FDA-regulated products are discovered within the supply chain by the manufacturer of a product or by the supplier's customers. When this occurs and a recall is needed, the agency requests that it be contacted by the company initiating the recall. FDA will typically email a few questions for the company involved, and will allow the company to prepare its own recall notices for its customers and for the FDA recall website (if it is a Class I recall). While executing most recalls of FDA-regulated products will tend to be a more pleasant experience than executing recalls of USDA-regulated products, there are nevertheless instances where, even on the FDA side, the decision to initiate a recall and define the ultimate scope are not always voluntary.
In 2024, both agencies became notably more aggressive when communicating to companies their own views on how broadly the scope of any given company's recall should be defined when pathogens are involved. In the past, both USDA and FDA would typically give a large amount of deference to food companies contemplating whether, and to what extent, to initiate a recall because of a concern with Listeria, for instance.
Last year, however, we saw some notable changes in how the agencies oversee recalls for Listeria concerns. In multiple examples, we observed both USDA and FDA demanding that certain companies recall all products they produced on multiple production lines during a given period, even when the Listeria concern was arguably limited to only a single Zone 1 issue on a single line.
The USDA and FDA approach of casting a wider net over production areas generally, as opposed to remaining more tightly focused on the specific line where an issue is discovered, is troubling. This approach also appears consistent with FDA's current definition of a "Zone 2" surface within the processing environment. Academia and industry—and, historically, regulators—have defined a Zone 2 surface as any surface immediately adjacent to a surface that comes into contact with a food. With that said, in FDA's 2024 Investigations Operations Manual, the agency defines "Zone 2" slightly differently for smaller processing rooms (e.g., under 20,000 square feet), to "…encompass all non-food contact surfaces in the processing area, such as the exterior of equipment, framework, food carts, equipment housing, gears, ventilation, air handling equipment, and floors." The definition is also very subjective for larger rooms, outlining Zone 2 to encompass the areas "…around the exposed product where you could envision a pathway to product contamination, either through the actions of people or machines." Such expansive definitions have seemingly allowed the agency to argue more recently that the scope of recalls be defined far more broadly.
When working with FDA during a recall, it is important to demand material information to aid the decision-making process, and to ask FDA questions about the agency's current thinking. In some cases, this interaction can favorably influence the ultimate decision about whether a recall is necessary and, if so, the appropriate scope and characterization.
Class Actions: A Rising Trend
Another trend we observed for the first time in 2024 was class action lawsuits being filed against food companies following their announcement of a food product recall. It is no secret that class action plaintiff lawyers are constantly looking for new theories upon which to bring lawsuits against food companies. In the latest iteration, a number of law firms have filed class action lawsuits against companies after they voluntarily recalled their food products for pathogen concerns, arguing that, because the company did not warn consumers that their products were potentially contaminated, the consumers were allegedly deceived into paying a higher premium for the product. These lawsuits typically seek reimbursement of the purchase price of the product, various penalties available to consumers who have allegedly been defrauded, and exorbitant attorneys' fees.
If these first few 2024 lawsuits targeting companies recalling food products for pathogens are successful, we anticipate that class action lawsuits will become a routine consequence for every company following any announcement of a voluntarily food product recall. It is also true that, for any food company, whether a recall may be required in the future is not a matter of "if," but, rather, a matter of "when." As a result, when faced with your first (or next) recall, be sure to consider the possible impact of a probable class action lawsuit and the need to dedicate substantial, uninsured resources to appropriately address and defend the same.
What Past Recall Trends Can Teach Us
So, what can we learn from studying past recalls? We can learn how to better avoid them in the future. If looking only at the number of total recalls last year, 2024 was a pretty "good" year. The number of total recalls published in 2024 was down significantly, at 491 recalls. This is down from the previous years of 2023, 2022, and 2021, during which saw 506, 504, and 532 total recalls, respectively.
In 2024, the leading driver of recalls was undeclared allergens, causing approximately 180 recalls. As is typical from year to year, pathogens were the second-largest driver of food product recalls. Listeria monocytogenes accounted for a total of 79 recalls, Salmonella accounted for 54 recalls, and pathogenic Escherichia coli accounted for an additional 14 recalls. In 2024, foreign material accounted for a total of 50 recalls. Metal, plastic, and glass were the three leading foreign materials causing recalls, with a handful of recalls announced for the presence of less desirable foreign materials, such as insects and rodent droppings.
Historically, over the past ten years, about 41 percent of all recalls have been driven by undeclared allergens, 26 percent of all recalls were driven by the presence of pathogens, and about 11 percent of all recalls were caused by foreign material. Table 1 displays the recall data from 2014–2024.1 It is fascinating that, from year to year, the overall percentages of recalls caused by undeclared allergens, pathogens, and foreign material have remained extremely consistent.
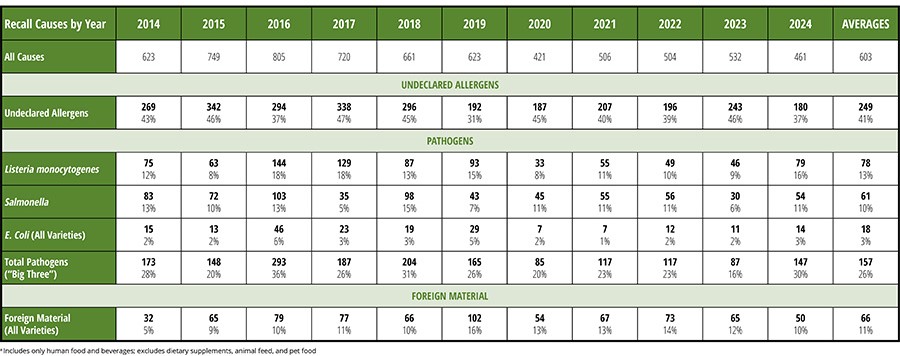
Even when we drill into the subcategories, such as the specific pathogens causing recalls of food products, the year-to-year data is quite consistent. Historically, year-to-year, Listeria has contaminated about 13 percent of all recalled products, Salmonella has contaminated about 10 percent of all recalled products, and pathogenic E. coli has contaminated about 3 percent of all recalled products. This should tell us that, overall, the food industry has not yet been overly successful in developing decisively effective controls for these recurring hazards.
Will the Recall Trends of 2024 Continue?
As we move deeper into 2025, in addition to the potential of broader and more expansive recalls and class action lawsuits, food companies will also continue to face the increased potential for criminal exposure in the event it is determined that their food products caused illness. In 2014, with the advent of new food safety rules and the passage of the Food Safety Modernization Act a few years prior, the U.S. Department of Justice (DOJ) announced publicly with FDA that it would no longer tolerate circumstances where food companies ship food products into interstate commerce that make people sick. Broadly speaking, DOJ has suggested that it would carefully review the circumstances underlying any foodborne illness outbreak to determine if there is a basis for criminal sanctions.
Over the years, we have observed DOJ focus on multiple companies, such as Chipotle, Blue Bell, and others, following foodborne illness outbreaks. To obtain a conviction against a company or a food safety professional working within that company, DOJ must prove that the defendant:
- Knew of a condition that could lead to consumers becoming sick (i.e., the discovery of intermittent Listeria findings in the processing environment through routine testing)
- Was in a position to eliminate the condition (i.e., completely halt production in the area of the positives, and then disassemble, clean, and sanitize the equipment)
- Failed to do so (i.e., usually evidenced by the fact of people getting sick and being linked to the outbreak and product).
If DOJ provides this proof, then a conviction could result in a fine (per count) of up to $250,000 USD, or one year in prison. I anticipate that DOJ may be involved in one or more of the recalls announced in 2024.
The world of recall exposure and management is changing, even as you read this article. The next time your company is faced with a potential recall decision, be sure that you are fully protecting your company and brand. Considering the recent trends discussed above, in the event you are asked or expected in the future to recall your products, make sure that you are approaching the challenge with the right training and information.
Reference
- Food Industry Counsel LLC. "Food Recall Reporter." https://www.foodindustrycounsel.com/recalls/.
Shawn Stevens, J.D. is the founding member of Food Industry Counsel LLC, the only law firm in the U.S. that represents the food industry exclusively. As a food industry consultant and lawyer, Mr. Stevens works with food industry clients (including the world's largest growers, processors, restaurant chains, distributors, and grocers), helping them protect their brand by complying with FDA and USDA regulations, reducing risk, managing recalls, and defending high-profile foodborne illness claims. Mr. Stevens also speaks regularly to national and international audiences on a wide variety of emerging scientific, regulatory, and food safety legal trends.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!





