Factoring Safety into Functional Foods

Diet, exercise, and eat right. This is the guiding tenet by which many health-conscious people live and one of the driving forces behind the popularity of functional foods. Despite their immense popularity, there is no universally accepted definition of functional foods. Nevertheless, functional foods can best be characterized as “any food or food ingredient that may provide a health benefit beyond the traditional nutrients it contains.”
Two years ago, functional food and beverage sales topped $25 billion in the United States alone. Propelled by expected growth in Eastern Europe, Western Europe and North America, the worldwide functional food market is expected to reach $90 billion in sales by 2013.
Asian Origins
The concept of functional foods can be traced back to the early 1900s when U.S. food manufacturers initiated the practice of adding iodine to salt to prevent goiter (an enlargement of the thyroid gland). Several dec-ades later, in the mid-1980s, the term was officially coined in Japan. Faced with a rapidly aging populace and rising health costs, the Japanese government championed the consumption of healthy food products to reduce risk factors associated with certain chronic diseases.
In 1991, the Japanese Ministry of Health, Labor and Welfare created the “Food for Specified Health Use” (FOSHU) standard, allowing functional food producers to make health claims on authorized product labels.
To achieve product approval from FOSHU, companies must undergo an intensive application process. Applications, which are reviewed by local prefecture authorities and the Ministry of Health and Welfare, must include scientific documentation demonstrating the particular basis for a health claim; the basis for the recommended dose of the functional ingredient; information demonstrating the safety of the ingredient; information on physical and chemical characteristics; relevant test methods; and a compositional analysis. Lastly, scientific papers must be submitted to substantiate the health claim.[1] By the end of last year, over 700 products had attained FOSHU approval in Japan.
Three’s Company
In their purest form, functional foods can be unprocessed fruits, vegetables or grain products or processed foods that utilize innovative, cutting-edge technologies. Functional foods, for the sake of simplicity, are generally grouped into three categories:
• Foods that have been fortified with specific nutrients to prevent deficiency-based diseases
• Foods that contain microorganisms or substances meant to promote the growth of microorganisms in the intestines
• Foods that contain bioactive components or enzymes (In some quarters, foods that have been enhanced through advanced science, such as special livestock feeding or genetic engineering, are also considered functional foods.)
Lions Roar
From vitamin-fortified cereals to chai beverages, functional foods have cut a wide and impressive path across the food industry landscape. PepsiCo, General Mills, Kellogg, Coca-Cola and Kraft are just a handful of the food manufacturing lions making hay in the functional foods market. Over the past several years, as competition has stiffened in this lucrative market, venerable and upstart companies are augmenting products with some familiar and novel ingredients to win customers and grow revenues.
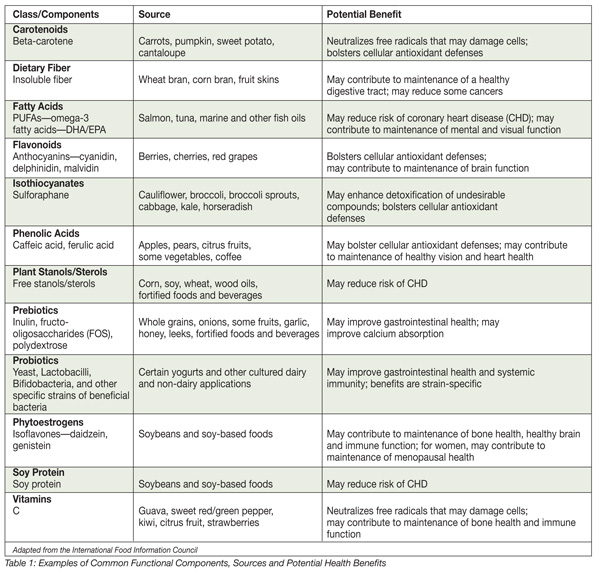
Omega-3s. Omega-3 fatty acids are long-chain polyunsaturated fatty acids that cannot be produced in the human body. Commonly referred to as essential fatty acids, docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) are found naturally in salmon, lake trout, tuna, herring and other cold-water fish. DHA is the most abundant fatty acid in the brain, and EPA is found in only small amounts. Omega-3 fatty acids have been found to be useful in reducing the risk of a host of diseases. According to many consumer surveys, more than half of the U.S. population has heard of omega-3 fatty acids and most associate them with a health benefit.
Soy isoflavones. Isoflavones are found in many foods, but are primarily derived from soybeans. The most important soy isoflavones are daidzein and genistein. Purported health benefits of these compounds include reduced risk of heart disease and protection against hormone-related disorders. Moreover, there are indications that isoflavones can stop the growth of cancer cells through inhibition of DNA replication and reduction in the activity of various enzymes.[2]
Green tea. Several industry and academic studies have found that green tea is more effective than both vitamins C and E in protecting cells against cellular damage. Purdue University researchers discovered green tea inhibits an enzyme required for cancer cell growth and can kill cultured cancer cells with no ill effect on healthy cells.[3]
Prebiotics. Prebiotics are found naturally in many foods and can be isolated from plants or synthesized For a food ingredient to be classified as a prebiotic, it must be demonstrated that it is i) not broken down in the stomach or absorbed in the GI tract, ii) fermented by the gastrointestinal microflora and, most importantly, iii) selectively stimulates the growth and/or activity of intestinal bacteria associated with health and well-being. The principal characteristic and effect of prebiotics in the diet is to promote the growth and proliferation of beneficial bacteria in the intestinal tract and, thus, potentially yield or enhance the effect of probiotic bacteria.
Probiotics. Better known as fermentative bacteria, probiotics can help control the growth of undesirable microorganisms, improve the immune response and aid in digestion. Studies have also explored possible anti-carcinogenic properties of probiotics.
Citrus fruits. Rich in antioxidants, studies have shown that citrus fruits are protective against a variety of human cancers. Citrus fruits are particularly high in a class of phytochemicals known as limonoids. Deemed “super fruits,” acai, goji, mangosteen, noni and other imports from exotic locales are increasingly being used in food and beverage products because of purported nutritional advantages.
Safety Factor
While there is evidence that some functional foods or ingredients can play a role in disease prevention and/or health promotion, the consideration of the safe use of these foods/ingredients is critical. For example, careful consideration should be given to the potential toxicity of increased uptake of selected nutrients. The Food and Nutrition Board of the National Academy of Sciences has devoted considerable attention to the upper safe limit for intake of essential nutrients.[4] As another example, the safety issues related to herbs are complex and the issue of herb-drug interaction has been receiving increased attention.[5]
In the IFT “Expert Report on Functional Foods,”[6] an expert panel stated that the safety assessment of functional foods should accept the components already considered through pre-established programs, such as generally recognized as safe (GRAS) substances and approved food additives. An objective, science-based evaluation process must establish that functional components are safe at their projected use levels. The report gives detailed information on safety testing for functional foods (in its Appendix D), with the most appropriate safety assessment needing to be determined on a case-by-case basis. Typically the safety assessment will include the following:
• Documented history of food use
• Estimates of current and proposed intakes of the functional component(s)
• Toxicological/safety assessment of new intake levels
Substances without a prior history of safe use will require a comprehensive and critical review of the scientific literature on the biological effects of the ingredient and on chemically related substances. Based on an initial review, specific studies will generally be required to define:6
• Bioavailability—likely modes of action in vivo
• Estimated half-life in vivo
• Estimated dose-response for a range of potential effects
• Known pharmacologic/toxic effect
• Evidence of allergenicity
• Toxicity and safety (human, experimental animals, in vitro systems, including microorganisms, cells in culture, and microarrays)
When the bioactive components of a functional food are not known, epidemiological studies on the safety of the whole food would be an important part of the assessment.
Under FDA guidelines, functional ingredients must be proven safe, require that claims be approved by the agency prior to marketing and prohibit companies from selling “functional foods” as dietary supplements or “medical foods.” Moreover, companies are charged with working with the U.S. Federal Trade Commission to develop a consistent policy for claims in advertising and labeling and require that labels list the amount of functional ingredients contained in a serving and, if appropriate, warning information.
John Williams Jr. is a Silliker, Inc. senior communications specialist and Cynthia Stewart, Ph.D., is General Manager of the Silliker Food Science Center, a leading U.S. research laboratory. The authors can be reached at info@silliker.com.
References:
1. Hasler, C. 2005. Regulation of Functional Foods and Nutraceuticals. Washington, DC: Wiley-Blackwell.
2. www.isoflavones.info/isoflavones-action.php
3. Coleman, P., and J. Williams Jr. 2007. Antioxidants: Scratching the Surface of Functional Foods. Food Product Design.
4. Milner, J. A. 1999. Functional Foods and Health Promotion. J Nutr 129:1395S-1397S.
5. Hasler, C. 2002. Benefits, Concerns and Challenges—A Position Paper from the American Council on Science and Health. J Nutr 132:3772-3781.
6. Institute of Food Technologists. 2005. IFT Expert Report on Functional Foods. Chicago: Institute of Food Technologists.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!