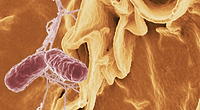
Targeting Biofilms with Cold Plasma: New Approaches to a Persistent Problem

When developing and maintaining an effective sanitation program for a food handling environment, you face a lot of challenges. These include the design of processing equipment for proper access to all surfaces for cleaning, selection of sanitizers and cleaning tools matched to the needs of each processing line, training for shift workers and cleaning crews, and the monitoring and verification of sanitation efficacy. For the food industry, however, almost nothing presents as many problems for antimicrobial sanitation as the difficulties presented by biofilms. First recognized as an issue for the food processing environment in the early 1990s and continuing through to the present day, biofilms represent, at best, a persistent harborage for native microbes and, at worst, a source of ongoing contamination with human pathogens, such as Salmonella, Shiga toxin-producing Escherichia coli and Listeria monocytogenes.[1–3] Ideally, a facility should be designed from floor to ceiling to reduce the potential for biofilm formation and use equipment designed for ready and effective cleaning. Even where conditions are ideal, however, biofilms remain a concern.
How Are Biofilms Organized?
 Biofilms are tightly grouped masses of microorganisms, clustered together in complex communities with concomitant extracellular polymeric substances excreted by the bacterial communities to provide water-impermeable cellular protection. Usually, when we think of bacteria, we imagine swarms of single cells. Nevertheless, most bacteria on earth live not as individual “planktonic” cells but within dense biofilm communities. Studies with single-species cultures have shown that bacterial isolates range in their ability to form biofilms, from those that readily create dense, robust clusters to those that are essentially biofilm-incapable.[4] However, many bacteria that would make weak biofilms by themselves will readily participate in the strong biofilms formed by other species. In fact, within naturally occurring biofilms, dozens of different species of bacteria interact in complex and as yet poorly understood ways. They share DNA, signal to each other, grow, reproduce and cooperate in metabolizing nearby nutrient sources. As the biofilm ages, different members of the microbial community will come to predominate, then fall back as others surge, driven by internal and external cues and changing conditions in the environment. Bacterial cells in a biofilm express hundreds of genes that are never turned on when those cells are in their free-floating planktonic state as individual bacteria. This genetic responsiveness makes biofilms and biofilm-associated microorganisms flexible and robust in how they respond to environmental stresses. Over time, a biofilm will start out as a very small mass, then attach to a surface, at first loosely, then very tightly. Once established, the biofilm expands, grows and eventually disperses into the environment (Figure 1[5]), sending a piece of itself off to colonize a new surface. Upon adhering to a new location, the biofilm can recruit additional microbial species from the environment, which can cause changes in the biofilm’s structure and function. The population dynamics within biofilms are only just now being mapped, and there are countless combinations of species mixtures, circumstances and environments to consider.
Biofilms are tightly grouped masses of microorganisms, clustered together in complex communities with concomitant extracellular polymeric substances excreted by the bacterial communities to provide water-impermeable cellular protection. Usually, when we think of bacteria, we imagine swarms of single cells. Nevertheless, most bacteria on earth live not as individual “planktonic” cells but within dense biofilm communities. Studies with single-species cultures have shown that bacterial isolates range in their ability to form biofilms, from those that readily create dense, robust clusters to those that are essentially biofilm-incapable.[4] However, many bacteria that would make weak biofilms by themselves will readily participate in the strong biofilms formed by other species. In fact, within naturally occurring biofilms, dozens of different species of bacteria interact in complex and as yet poorly understood ways. They share DNA, signal to each other, grow, reproduce and cooperate in metabolizing nearby nutrient sources. As the biofilm ages, different members of the microbial community will come to predominate, then fall back as others surge, driven by internal and external cues and changing conditions in the environment. Bacterial cells in a biofilm express hundreds of genes that are never turned on when those cells are in their free-floating planktonic state as individual bacteria. This genetic responsiveness makes biofilms and biofilm-associated microorganisms flexible and robust in how they respond to environmental stresses. Over time, a biofilm will start out as a very small mass, then attach to a surface, at first loosely, then very tightly. Once established, the biofilm expands, grows and eventually disperses into the environment (Figure 1[5]), sending a piece of itself off to colonize a new surface. Upon adhering to a new location, the biofilm can recruit additional microbial species from the environment, which can cause changes in the biofilm’s structure and function. The population dynamics within biofilms are only just now being mapped, and there are countless combinations of species mixtures, circumstances and environments to consider.
How Can We Remove Biofilms?
What makes biofilms such a headache for sanitation programs is the way they’re structured. All of these closely interacting cells are attached to surfaces by a kind of naturally occurring superglue, a polymer matrix made of cross-linked extracellular polymeric substances (EPS). This EPS not only locks the biofilm in place but also shelters the microbial community from the environment, protecting and preserving it. In natural settings, such as streams and ponds, the EPS protects the bacteria from desiccation, temperature shifts, osmotic shocks and other environmental stresses. In artificial structural settings—drains, pipes, floors, belts, etc.—the EPS matrix also inhibits the antimicrobial efficacy of chlorinated sanitizers, organic acids and other chemical tools. Some biofilm-associated microorganisms may require up to a 600-fold increase in chlorine concentration to achieve the same lethality that the antibacterial retains against planktonic cells.[6,7] Recent research suggests that repeated cleaning cycles in food processing environments can select for genetic variants of L. monocytogenes and other microorganisms that possess the genes necessary to tolerate quaternary ammonium sanitizers.[8] Novel strategies are being pursued to address biofilms in the food industry. Some of these, such as enzyme-based detergents or quorum-sensing antagonists, enhance the efficacy of conventional sanitizers by dispersing the bacterial cells from their protective biofilm. Others, such as bacteriophage and bacteriocin treatments, seek to more effectively kill the biofilm-associated bacteria in place, penetrating the protective EPS matrix to reach the cells within.[9] Among the novel physical processes being evaluated for efficacy against biofilms is cold plasma.
Is Cold Plasma a Biofilm Silver Bullet?
Cold plasma is a form of ionized gas. Unlike flames, electric arcs or other more familiar kinds of plasmas, which can be hundreds of degrees hotter than ambient temperatures, the cold plasmas being developed for use in food processing operate at close to room temperature. They are a nonthermal food safety intervention, in the sense that they do not rely on heat for microbial inactivation.[10] Cold plasmas are extremely reactive and are made by energizing pure gases or gas mixtures with high-voltage electricity. The electric charge strips gas molecules apart, creating ions, free electrons, oxygen singlet atoms, reactive radical species and other gas plasma products. The antimicrobial modes of action of cold plasma arise from chemical reactions of these plasma-reactive particles and molecules with bacterial cell structures and from additional ultraviolet (UV) damage to DNA and other cellular components caused by a UV light component of the cold plasma. Dozens of cold plasma technologies are being actively developed and evaluated around the world. Each has its own strengths, which are, in many cases, closely aligned to the specific contamination problems at hand. A growing body of literature is evaluating cold plasma as a tool for killing Salmonella, pathogenic E. coli, L. monocytogenes, norovirus and other pathogens on a wide variety of foods and food contact surfaces. These studies have shown that multi-log reductions of pathogens are possible, and cold plasma is increasingly seen as a promising technology for various food processing applications.[11]
With respect to biofilms, cold plasma is already gaining attention as a potential tool with applications in the medical field where biofilms are a problem, including in dental and oral treatments.[12] Drawing on that body of research, and leveraging the knowledge obtained, will significantly hasten commercialization of the technology for applications in the food industry. However, while much can be learned from these studies, there are some important distinctions to be made among the various cold plasma technologies under development, based on their likely suitability for crossover commercialization. In a food processing plant, biofilms are environmental—they form in the less-accessible sections of drains, on the surfaces of equipment, in areas where standing water is a problem, in hard-to-reach microbiological niches, etc. To be effective, a cold plasma treatment would need to be able to reach these areas. This would tend to limit the utility of cold plasmas created inside vacuum chambers and plasmas created using noble gases such as helium, argon and neon. While these gases ionize at lower voltages than air, nitrogen or oxygen, the reduced electricity needs are offset by the cost of the feed gas. The economics of cold plasma scale-up were shown to depend heavily on the composition of the feed gas and its cost.[10] For example, a gas plasma system based on a 99 percent helium mixture would face one set of economic constraints for use in a dentist’s office to sterilize a root canal but would face a very different set of criteria for use on a large scale to sanitize equipment, tables, belts, etc. during an end-of-shift cleaning cycle at a food processing facility. Although a large body of literature exists on cold plasmas generated in partial vacuums and using noble gases, the research discussed in this article will be limited to plasmas generated at atmospheric pressure using air or oxygen/nitrogen gas mixtures, except where noted.
How Would Cold Plasma Work in Sanitation?
 Part of what makes cold plasma a potentially exciting technology for sanitizing is that the only major inputs to the system are air and electricity. As a waterless process that does not involve conventional sanitizers, such as chlorine compounds, organic acids, etc., cold plasma is seen as a “green” option for addressing biofilm contamination. Research has been published evaluating the antimicrobial efficacy of cold plasma as applied to pathogen biofilms grown on glass, a common model material for food contact surfaces. In one study, corona discharges were applied to 48-hour-old E. coli biofilms grown on glass slides. A reduction of up to 5 logs was seen after exposure for 15 minutes.[13] The overall killing efficiency of the cold plasma was enhanced by introducing a small amount of water onto one of the corona electrodes, which entered the plasma mixture in the form of an electrospray—a fine mist of electrically charged droplets. One important finding from this study was that the antimicrobial efficacy of the plasma was seen mostly in the upper layers of the biofilm. While cells on the top layers were killed, cells inside the biofilm were not as affected. This result suggests that the mechanisms of inactivation for this cold plasma system were primarily chemical in nature and that the reactive chemical species of the cold plasma were responsible for killing the E. coli, rather than a radiative UV component. Another key finding was that in experiments incorporating electrosprayed water with the corona discharge, the biofilm thickness was reduced. Approximately 35 percent of the biofilm was eroded after a 15-minute treatment. Because no mechanical scrubbing or abrasion was part of this study, this is an indication that cold plasma can break down the physical structure of the protective EPS that holds the biofilm in place. This degradation of the EPS matrix was also seen in another study using Salmonella biofilms subjected to corona discharges (unpublished data). Pathogen biofilms were grown on glass slides for 24 hours, then placed inside polyethylene terephthalate food containers. After exposure to a corona-discharge cold plasma for 3 minutes, the EPS structure of the biofilm was degraded and dispersed (Figure 2). In yet another study, cold plasma was applied to a three-strain Salmonella biofilm, grown for either 24, 48 or 72 hours, to determine the effect of biofilm age and maturity on its ability to resist the sanitizing effects of cold plasma.14 Using a model conveyor belt system, the contaminated test surfaces were treated with a cold plasma jet, a device that uses forced air to blow the cold plasma onto the surface to be treated (Figure 3). Treatments in this study lasted 5, 10 or 15 seconds. Even at such short treatment times, cold plasma reduced Salmonella biofilms by up to 1.57, 1.82 and 2.13 logs for the 5-, 10- and 15-second treatments, respectively. These reductions were seen for all the Salmonella biofilms, regardless of biofilm age or maturity. The authors concluded that this plasma jet system could have applications for food contact surfaces such as belts, prep areas, equipment, etc., especially if combined with a mechanical scrubbing process.
Part of what makes cold plasma a potentially exciting technology for sanitizing is that the only major inputs to the system are air and electricity. As a waterless process that does not involve conventional sanitizers, such as chlorine compounds, organic acids, etc., cold plasma is seen as a “green” option for addressing biofilm contamination. Research has been published evaluating the antimicrobial efficacy of cold plasma as applied to pathogen biofilms grown on glass, a common model material for food contact surfaces. In one study, corona discharges were applied to 48-hour-old E. coli biofilms grown on glass slides. A reduction of up to 5 logs was seen after exposure for 15 minutes.[13] The overall killing efficiency of the cold plasma was enhanced by introducing a small amount of water onto one of the corona electrodes, which entered the plasma mixture in the form of an electrospray—a fine mist of electrically charged droplets. One important finding from this study was that the antimicrobial efficacy of the plasma was seen mostly in the upper layers of the biofilm. While cells on the top layers were killed, cells inside the biofilm were not as affected. This result suggests that the mechanisms of inactivation for this cold plasma system were primarily chemical in nature and that the reactive chemical species of the cold plasma were responsible for killing the E. coli, rather than a radiative UV component. Another key finding was that in experiments incorporating electrosprayed water with the corona discharge, the biofilm thickness was reduced. Approximately 35 percent of the biofilm was eroded after a 15-minute treatment. Because no mechanical scrubbing or abrasion was part of this study, this is an indication that cold plasma can break down the physical structure of the protective EPS that holds the biofilm in place. This degradation of the EPS matrix was also seen in another study using Salmonella biofilms subjected to corona discharges (unpublished data). Pathogen biofilms were grown on glass slides for 24 hours, then placed inside polyethylene terephthalate food containers. After exposure to a corona-discharge cold plasma for 3 minutes, the EPS structure of the biofilm was degraded and dispersed (Figure 2). In yet another study, cold plasma was applied to a three-strain Salmonella biofilm, grown for either 24, 48 or 72 hours, to determine the effect of biofilm age and maturity on its ability to resist the sanitizing effects of cold plasma.14 Using a model conveyor belt system, the contaminated test surfaces were treated with a cold plasma jet, a device that uses forced air to blow the cold plasma onto the surface to be treated (Figure 3). Treatments in this study lasted 5, 10 or 15 seconds. Even at such short treatment times, cold plasma reduced Salmonella biofilms by up to 1.57, 1.82 and 2.13 logs for the 5-, 10- and 15-second treatments, respectively. These reductions were seen for all the Salmonella biofilms, regardless of biofilm age or maturity. The authors concluded that this plasma jet system could have applications for food contact surfaces such as belts, prep areas, equipment, etc., especially if combined with a mechanical scrubbing process.
 Results from these studies highlight some of the important aspects of cold plasma research deserving careful attention. All of these studies utilized atmospheric cold plasmas based on air, without mechanical abrasion or scrubbing steps, and all examined biofilms of comparable ages, which were grown on the same substrate (glass surfaces). However, they used different cold plasma technologies (corona discharge versus plasma jet), different pathogens (E. coli versus Salmonella) and obtained different levels of reductions, ranging from more than 5 logs in 15 minutes13 to 2.13 logs in 15 seconds.[14] Will a greater degree of biofilm reduction justify a longer treatment time? Will a faster process make moderate antimicrobial efficacy a more practical and economically feasible tool? As various types of cold plasma systems are optimized in different ways, these criteria will help guide the research and development of this technology.
Results from these studies highlight some of the important aspects of cold plasma research deserving careful attention. All of these studies utilized atmospheric cold plasmas based on air, without mechanical abrasion or scrubbing steps, and all examined biofilms of comparable ages, which were grown on the same substrate (glass surfaces). However, they used different cold plasma technologies (corona discharge versus plasma jet), different pathogens (E. coli versus Salmonella) and obtained different levels of reductions, ranging from more than 5 logs in 15 minutes13 to 2.13 logs in 15 seconds.[14] Will a greater degree of biofilm reduction justify a longer treatment time? Will a faster process make moderate antimicrobial efficacy a more practical and economically feasible tool? As various types of cold plasma systems are optimized in different ways, these criteria will help guide the research and development of this technology.
In addition to simulated food contact surfaces, cold plasma has been tested on pathogen biofilms grown directly on foods. Lettuce leaves were dip-inoculated in cultures of Salmonella Typhimurium, L. monocytogenes or E. coli and incubated for up to 48 hours to allow biofilms to form.[15] They were then packaged and treated with a form of atmospheric cold plasma known as “afterglow,” a mixture of the longest-lived cold plasma reaction products. “Active” plasma is rich in free electrons, oxygen singlets, reactive radical molecules and other very short-lived products of plasma ionization. In the time it takes to travel from the high-voltage electrodes to the bacterial target, some of these recombine to make the chemical species that predominate in “afterglow” cold plasma: O3, H2O2, NOX, etc. The leaf-associated biofilms treated with this type of cold plasma showed reductions of up to 5 logs after a 5-minute treatment. The efficacy of the process depended on pathogen type, storage conditions and age of the biofilm. It is worth noting that the same treatment, applied to free-living, planktonic cells, elicited nearly complete reductions in approximately 30 seconds. This highlights the relative durability and resistance of biofilm-associated pathogens, even to cold plasma treatments, but also suggests that cold plasma can be an effective antimicrobial process for biofilms on actual food products, not just on food contact surfaces.
Where Do We Go from Here?
For effective application of cold plasma to reduce biofilms in the food industry, this technology will need to be validated as a robust and reliable process. Since cold plasma is essentially a dry, nonthermal, chemical-free technology, it offers certain potential advantages over conventional wash systems. In environments with water restrictions, or in situations where water usage incurs charges for usage and/or wash water discharge volumes (so-called pump-and-dump fees), a dry sanitation system could have economic applicability for some anti-biofilm cleaning processes. Further, since cold plasma does not require the use of chlorinated compounds, organic acids or other conventional chemical agents and leaves behind no residue, it may play a greater role in continuous cleaning applications for food handling equipment in food processing systems. This could be of particular interest where a low-input, more sustainable sanitation technology would be desired. As cold plasma technologies are developed, they must be validated to ensure efficacy, functionality, flexibility and feasibility in the types of real-world settings and conditions that are of greatest concern to the food industry. The most likely case would be combining cold plasma with conventional sanitizing strategies as an integrated solution to effectively deal with microbial biofilms across the entire landscape of food processing equipment, facilities and products.
The author thanks Drs. Joshua Gurtler and George Paoli for their reviews of this article.
Brendan A. Niemira, Ph.D., is the Research Leader of the Food Safety and Intervention Technologies Research Unit, U.S. Department of Agriculture, Agricultural Research Service.
References
1. Carpentier, B and O Cerf. 1993. “Biofilms and Their Consequences, with Particular Reference to Hygiene in the Food Industry.” J Appl Bacteriol 75:499–511.
2. Langsrud, S et al. 2016. “Microbial Dynamics in Mixed Culture Biofilms of Bacteria Surviving Sanitation of Conveyor Belts in Salmon-Processing Plants. J Appl Microbiol 120:366–378.
3. Sharma, M and SK Anand. 2002. “Biofilms Evaluation as an Essential Component of HACCP for Food/Dairy Processing Industry — A Case.” Food Control 13:469–477.
4. Solomon, EB et al. 2005. “Biofilm Formation, Cellulose Production and Curli Biosynthesis by Salmonella spp. Originating from Produce, Animal and Clinical Sources.” J Food Prot 68(5):906–912.
5. commons.wikimedia.org/w/index.php?curid=3364284.
6. Luppens, SBI et al. 2002. “Development of a Standard Test to Assess the Resistance of Staphylococcus aureus Biofilm Cells to Disinfectants.” Appl Environ Microbiol 68:4194–4200.
7. Niemira, BA and P Cooke. 2010. “Escherichia coli O157:H7 Biofilm Formation on Lettuce and Spinach Leaf Surfaces Reduces Efficacy of Irradiation and Sodium Hypochlorite Washes.” J Food Sci 75(5):M270–M277.
8. Møretrø, T et al. 2017. “Tolerance to Quaternary Ammonium Compound Disinfectants May Enhance Growth of Listeria monocytogenes in the Food Industry.” Int J Food Microbiol 241:215–224.
9. Coughlan, LM et al. 2016. “New Weapons to Fight Old Enemies: Novel Strategies for the (Bio)control of Bacterial Biofilms in the Food Industry.” Front Microbiol 7:1641.
10. Niemira, BA. 2012. “Cold Plasma Decontamination of Foods.” Annu Rev Food Sci Technol (3):125–142.
11. Misra, NN et al. Cold Plasma in Food and Agriculture (New York: Academic Press, 2016).
12. Brelles-Mariño, G. 2012. “Challenges in Biofilm Inactivation: The Use of Cold Plasma as a New Approach.” J Bioprocess Biotech 2:e107.
13. Kovalova, Z et al. 2016. “Corona Discharges with Water Electrospray for Escherichia coli Biofilm Eradication on a Surface.” Bioelectrochem 112:91–99.
14. Niemira, BA et al. 2014. “Cold Plasma Rapid Decontamination of Food Contact Surfaces Contaminated with Salmonella Biofilms.” J Food Sci 79(5):M917–M922.
15. Ziuzina, D et al. 2015. “Cold Plasma Inactivation of Internalised Bacteria and Biofilms for Salmonella enterica serovar Typhimurium, Listeria monocytogenes and Escherichia coli.” Int J Food Microbiol 210:53–61.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!