Essentials of Hazard Analysis for Process Preventive Controls: Part 2

In Part 1 of our article series, we talked about the basic principles of Hazard Analysis and Critical Control Points (HACCP) and the regulations specific to agencies in the United States [U.S. Food and Drug Administration (FDA) and U.S. Department of Agriculture (USDA)] and Canada (Canadian Food Inspection Agency).
An outcome of a manufacturing facility’s Hazard Analysis (based on the guidance discussed in Part 1) would include identification of preventive controls that would significantly minimize and prevent the identified reasonably foreseeable hazards. These preventive controls can be broadly classified into process controls, food allergen controls, sanitation controls, and supply chain controls as appropriate to the nature of the facility and the products produced. This article focuses on the requirements for the identification and implementation (specifically the preventive control management components: monitoring, corrective actions, verification and records) of process preventive controls.
Process Preventive Controls
Per the Food Safety Modernization Act Section 117.135, process preventive controls include “procedures, practices, and processes to ensure the control of parameters during operations such as heat processing, acidifying, irradiating, and refrigerating foods. Process controls must include, as appropriate to the nature of the applicable control and its role in the facility’s food safety system:
(i) Parameters associated with the control of the hazard; and
(ii) The maximum or minimum value, or combination of values, to which any biological, chemical, or physical parameter must be controlled to significantly minimize or prevent a hazard requiring a process control.”
 Process preventive controls play an important role in a facility’s food safety plan since they are considered very critical for food safety. Process preventive controls (e.g., thermal processing, irradiation) result in significant reduction in potential food safety hazards and hence are often deemed CCPs (CCPs require establishment of critical limits) to reinforce the importance of their role in a facility’s food safety plan. In some situations, process preventive controls, where the parameters (referred to as quality control points or operational limits) required to produce a saleable acceptable product quality far exceed the established food safety limits, may be managed as an operational prerequisite program (OPRP) in a facility’s safety plan. In this situation, the food safety team must perform a scientific risk assessment in conjunction with expert microbiologists and statisticians to justify the decision (Figure 1). One common mistake in determining CCPs/OPRPs for the food safety team is to start with existing controls and determine which of these are CCPs/OPRPs, ignoring the Hazard Analysis. This will probably result in over- or underestimating the reasonably foreseeable hazards, leading to poor identification of preventive controls and thereby resulting in an inadequate food safety plan. Decision trees provide a meaningful and standardized approach to help the manufacturer identify if the specific process preventive control can be managed as a CCP or OPRP. The decision to choose a CCP or OPRP must be science-based and be performed in consultation with a subject matter expert (SME) and not simply refer to past decisions and/or current facility procedures. Following the decision of the choice of CCP or OPRP, the food safety team must then list parameters and critical limits/operational limits (or quality control points, the minimum or maximum values associated with the parameters) for the controls for each hazard.
Process preventive controls play an important role in a facility’s food safety plan since they are considered very critical for food safety. Process preventive controls (e.g., thermal processing, irradiation) result in significant reduction in potential food safety hazards and hence are often deemed CCPs (CCPs require establishment of critical limits) to reinforce the importance of their role in a facility’s food safety plan. In some situations, process preventive controls, where the parameters (referred to as quality control points or operational limits) required to produce a saleable acceptable product quality far exceed the established food safety limits, may be managed as an operational prerequisite program (OPRP) in a facility’s safety plan. In this situation, the food safety team must perform a scientific risk assessment in conjunction with expert microbiologists and statisticians to justify the decision (Figure 1). One common mistake in determining CCPs/OPRPs for the food safety team is to start with existing controls and determine which of these are CCPs/OPRPs, ignoring the Hazard Analysis. This will probably result in over- or underestimating the reasonably foreseeable hazards, leading to poor identification of preventive controls and thereby resulting in an inadequate food safety plan. Decision trees provide a meaningful and standardized approach to help the manufacturer identify if the specific process preventive control can be managed as a CCP or OPRP. The decision to choose a CCP or OPRP must be science-based and be performed in consultation with a subject matter expert (SME) and not simply refer to past decisions and/or current facility procedures. Following the decision of the choice of CCP or OPRP, the food safety team must then list parameters and critical limits/operational limits (or quality control points, the minimum or maximum values associated with the parameters) for the controls for each hazard.
Validation is a key component in the design of critical limits/operational limits for process preventive controls. In recent years, the requirement for thermal process validations has received significant attention owing to several recalls of low- moisture foods.[1–6] Thermal process validation approaches may typically be classified into three categories:[7]
1. Measurement of the physical delivery of the process and comparison with published data
2. A microbiological challenge study of the process with pathogen strains or a valid surrogate organism, to demonstrate a desired reduction
3. Process modeling with data from thermal-death time studies, using data either from the literature or from experiments conducted by the processor
Similar approaches can be applied for other process preventive controls such as irradiation, acidification, and refrigeration of foods. There is a plethora of information regarding how to perform validation studies for different types of process preventive controls from several credible resources.[8–12]
Finally, the elements of monitoring, corrective actions to be taken when deviations from the critical/operational limits occur, verification procedures, and records must also be documented as part of the food safety plan.
Monitoring: Monitoring is defined as “the act of conducting a planned sequence of observations or measurements of control parameters to assess whether a control measure is under control” (21 C.F.R. 117.3, Definitions).
 Monitoring of CCPs/OPRPs must be routinely conducted to determine whether the process is operating within the critical/operational limits. Appropriate corrective actions must be taken when deviations relating to critical limits/operational limits are encountered. Monitoring activities should be designed to alert the designated employee/qualified individual conducting the monitoring activities in the event of a deviation. As outlined by the National Advisory Committee on Microbiological Criteria for Foods (NACMCF),9 monitoring serves three main purposes: 1) tracking of the operation; 2) determining when there is a loss of control or when a deviation from a critical limit/operational limit occurs; and 3) monitoring activities that involve measurement and/observation. Monitoring differs from verification in that it is intended to provide real-time data on whether a CCP/OPRP is implemented properly. There are four elements of monitoring: 1) what will be monitored; 2) how it will be monitored; 3) when it will be monitored; and 4) who will perform the monitoring.
Monitoring of CCPs/OPRPs must be routinely conducted to determine whether the process is operating within the critical/operational limits. Appropriate corrective actions must be taken when deviations relating to critical limits/operational limits are encountered. Monitoring activities should be designed to alert the designated employee/qualified individual conducting the monitoring activities in the event of a deviation. As outlined by the National Advisory Committee on Microbiological Criteria for Foods (NACMCF),9 monitoring serves three main purposes: 1) tracking of the operation; 2) determining when there is a loss of control or when a deviation from a critical limit/operational limit occurs; and 3) monitoring activities that involve measurement and/observation. Monitoring differs from verification in that it is intended to provide real-time data on whether a CCP/OPRP is implemented properly. There are four elements of monitoring: 1) what will be monitored; 2) how it will be monitored; 3) when it will be monitored; and 4) who will perform the monitoring.
What will be monitored: Depending on the nature of the control measure, monitoring activities may include (but are not limited to) measurement of pH, temperature, time, volume/weight, flow rate, acid addition, water activity, chemical concentration, appearance, process performance, and other relevant factors as appropriate to the control measure.
How it will be monitored: Selecting an appropriate monitoring device should be the first order of business when designing monitoring activities. Monitoring activities are expected to produce an accurate record for future use in verification; hence, the measurement monitoring devices should be calibrated and deliver highly sensitive and accurate measurements.
When it will be monitored: Monitoring activities may be classified into continuous or noncontinuous. For noncontinuous monitoring activities, it is important to perform at a frequency sufficient to establish process control. Process capability studies may be considered to determine the appropriate frequency. Continuous monitoring at a CCP/OPRP is preferred but may not always be practical or necessary. Continuous monitoring becomes more of a critical requirement if minor variations/deviations in the critical limits may otherwise go unnoticed. Automatic and continuous monitoring is possible with many types of physical (temperature, time) and chemical measurements (pH, chlorine concentration). In some cases, modern technology has made it possible to continuously monitor variables like temperatures on statistical process control charts and electronically communicate (email alerts) to designated employee(s)/qualified individual(s) where deviations from critical/operational limits are encountered. This will not only facilitate real-time verification of a facility’s food safety system but also trigger corrective actions in a timely manner.[13]
Who will perform the monitoring: Qualified individuals assigned to preventive controls monitoring activities must receive appropriate training for the tasks.
Corrective actions: An important purpose of corrective actions is to prevent adulterated foods from entering commerce. Where there is a deviation from established critical/operational limits, corrective actions are necessary. Therefore, corrective actions should a) identify the problem; b) correct or contain the problem; c) evaluate affected food for safety and prevent it from entering commerce if you cannot ensure (prove and document) the affected food is not adulterated or misbranded; d) determine the root cause of the nonconformance; e) take corrective action to prevent its recurrence; f) identify and initiate preventive actions to eliminate the cause for the future (i.e., proactive procedure); g) review and approve the corrective and preventive action report; h) monitor and evaluate the corrective and preventive actions to ensure that they are effective; i) when appropriate, reanalyze the food safety plan to determine whether modification of the plan is required; and j) document all actions performed. Specific corrective actions should be developed in advance for each CCP/OPRP and included in the facility’s food safety plan. At a minimum, the food safety plan should identify what was done in the event of deviation and identify the role and responsibility of the person performing the activity. Individuals who have thorough understanding of the process, product, and food safety plan should be assigned the responsibility for oversight of corrective actions. As appropriate, SMEs may be consulted to perform a scientific evaluation to determine the disposition of the nonconforming product.[13]
Verification: The primary purpose of the verification activity is to evaluate if a facility’s food safety system is effectively functioning as intended. An effective food safety system may require little product testing, since it relies on frequent reviews of their food safety plan, effective management of preventive controls, and foundational food safety programs (PRPs). Another aspect of verification is the initial validation of the food safety plan to determine that the plan is scientifically and technically sound, that all hazards have been identified, and that if the food safety plan is properly implemented, these hazards will be effectively controlled. Information needed to validate the food safety plan often includes expert advice and scientific studies, and in-plant observations, measurements, and evaluations. For example, validation of the roasting process of peanuts must include the scientific justification of the heating times and temperatures needed to obtain an appropriate destruction of pathogenic microorganisms (e.g., Salmonella) and studies to confirm that the conditions of roasting will deliver the required time and temperature to each peanut. Subsequent validations are triggered by major changes to the food safety plan (e.g., change of process, use of new raw material supplier, emergence of new hazards, installation of new equipment).[13]
Records: Records maintained for the food safety system should include the following: 1) a summary of the Hazard Analysis, including the rationale for determining hazards and control measures, and 2) CCP/OPRP summary tables identifying hazards of concern, critical/operational limits, monitoring, corrective actions, verification procedures, and record-keeping procedures.
In this article, we describe two examples to illustrate how Hazard Analysis drives the decision to choose a CCP/OPRP and eventually develop preventive control management components (monitoring, verification, corrective actions, and record keeping).
Example 1: High-moisture fishery products in which temperature is the sole requirement to prevent toxin formation and is managed as a CCP in a facility’s food safety plan.
Note: Example 1 is provided for illustration purposes only based on the guidance published in Fish and Fishery Products Hazards and Controls Guidance.[14] Manufacturing facilities must perform Hazard Analysis and identify preventive controls as appropriate to the nature of the applicable control and its role in the facility’s food safety system.
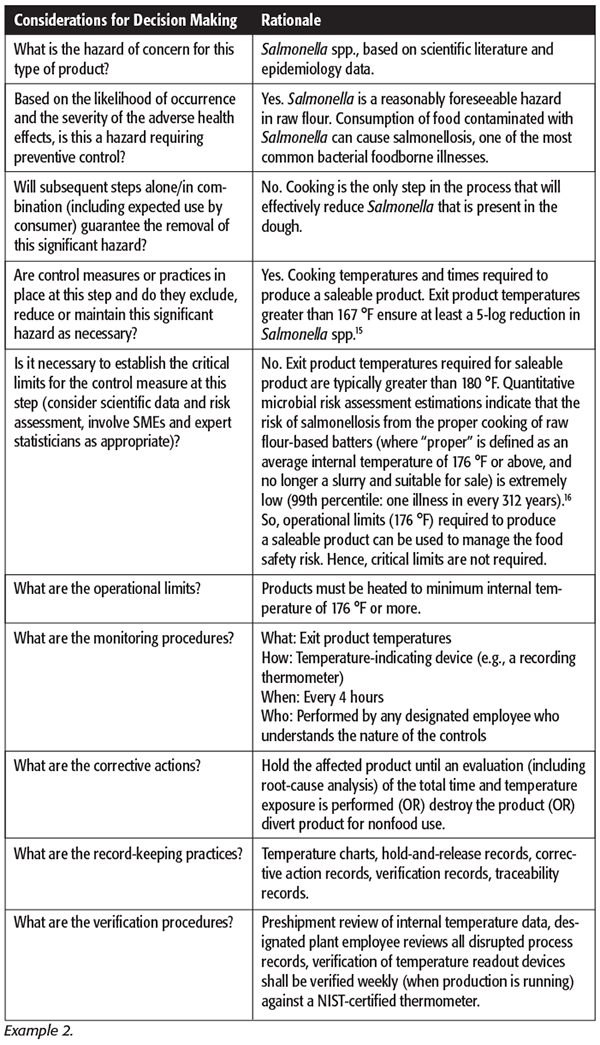 Example 2: Frozen bakery products in which a cooking process is used to significantly minimize or prevent Salmonella spp. survival and is managed as an OPRP in a facility’s food safety plan.
Example 2: Frozen bakery products in which a cooking process is used to significantly minimize or prevent Salmonella spp. survival and is managed as an OPRP in a facility’s food safety plan.
Note: The above example is provided for illustration purposes only based on the International Association for Food Protection (IAFP) poster presentations by Kottapalli et al.[15] and Kottapalli and Schaffner.[16] Manufacturing facilities must perform Hazard Analysis and identify preventive controls as appropriate to the nature of the applicable control and its role in the facility’s food safety system.
Advantages, Disadvantages, and Limitations of Tools
The main advantage of using decision trees and/or risk matrices is that they provide a straightforward, easily documented approach to determining the type of control measure needed to address an identified hazard during risk analysis. For larger companies with multiple facilities, these tools are a good way to standardize the Hazard Analysis approach across the company. Codex Alimentarius recommends training in the use of the decision tree,[17] and in the Seafood HACCP guidance, FDA recommends that users “not rely exclusively on the decision tree, because error may result.”[14] Each facility and food safety plan is different and requires expert review to ensure the decision tree results are, in fact, appropriate to the Hazard Analysis. Some examples of this limitation are described below.
Earlier decision trees developed prior to the introduction of the preventive control concept have an inherently limited flexibility to consider OPRPs. These early trees have only “CCP” and “Not a CCP” options and do not describe what needs to happen for those control measures deemed “Not a CCP.” While the assumption is that the “Not a CCP” measure will be maintained as a Good Hygiene Practice (GHP) or PRP, this is not explicitly stated within the decision trees themselves. Use of the modified decision trees in Figure 1 could be more helpful to facilities that have preventive controls to be managed as OPRPs. The Codex Committee on Food Hygiene is considering adding the concept of “Control Measures at Places other than CCPs” or “Enhanced GHPs,” but this terminology has not yet been finalized.[18] There is a proposed decision tree (Figure 2) that accompanies this revision.
 While the risk matrix approach may seem a good quantitative technique, the Hazard Analysis becomes more difficult when it gets to the point of assigning number rankings to certain hazards. Questions can arise on how much of a dose is considered a high dose, what is really the difference between a consumer who is ill and one who goes to the hospital, and what is the difference between something that occurs occasionally, occurs often, or is a common occurrence? Another option could be to take a more conservative approach to risk analysis, and instead of assigning number rankings to severity and likelihood, the decisions are made as to whether the hazard is or isn’t severe, and whether the hazard is or isn’t likely to occur. In this case, severity and likelihood rankings are listed simply as Yes or No. Combinations of Yes and No will determine whether the hazard is one that needs a preventive control and what type of preventive control it should be (Table 1).
While the risk matrix approach may seem a good quantitative technique, the Hazard Analysis becomes more difficult when it gets to the point of assigning number rankings to certain hazards. Questions can arise on how much of a dose is considered a high dose, what is really the difference between a consumer who is ill and one who goes to the hospital, and what is the difference between something that occurs occasionally, occurs often, or is a common occurrence? Another option could be to take a more conservative approach to risk analysis, and instead of assigning number rankings to severity and likelihood, the decisions are made as to whether the hazard is or isn’t severe, and whether the hazard is or isn’t likely to occur. In this case, severity and likelihood rankings are listed simply as Yes or No. Combinations of Yes and No will determine whether the hazard is one that needs a preventive control and what type of preventive control it should be (Table 1).
 Depending on the situation at the individual facility, there are additional things to consider when determining the type of preventive control. The company or manufacturing facility food safety culture may be such that employees are biased toward “CCP thinking” and “prerequisite thinking.” In these situations, operators are used to managing only two types of controls—either it is critical or it is not. Without intensive and appropriate training, having an OPRP control could cause confusion and result in less than optimal monitoring and verification of the control, since operators may not be able to discern the difference between an OPRP and the PRPs they are used to implementing. For facilities that operate under multiple regulatory jurisdictions, again there are opportunities for confusion among floor operators. For example, in a facility that makes snack kits, some of which contain meat and some that do not, a particular preventive control needs to be an OPRP in the FDA HACCP plan for the meatless snack kits, and a PRP in the USDA plan for the meat-containing snack kits. The monitoring and verification activities could be performed at different frequencies, and corrective actions could be different. Facilities that are audited under any of the various Global Food Safety Initiative auditing schemes will need to justify their decision-making process for the choice of preventive controls, so a well-documented procedure to follow when making these decisions is a necessary component of an HACCP/food safety plan. Facility personnel will need to understand and be able to explain any and all tools that were used to determine the type of preventive control.
Depending on the situation at the individual facility, there are additional things to consider when determining the type of preventive control. The company or manufacturing facility food safety culture may be such that employees are biased toward “CCP thinking” and “prerequisite thinking.” In these situations, operators are used to managing only two types of controls—either it is critical or it is not. Without intensive and appropriate training, having an OPRP control could cause confusion and result in less than optimal monitoring and verification of the control, since operators may not be able to discern the difference between an OPRP and the PRPs they are used to implementing. For facilities that operate under multiple regulatory jurisdictions, again there are opportunities for confusion among floor operators. For example, in a facility that makes snack kits, some of which contain meat and some that do not, a particular preventive control needs to be an OPRP in the FDA HACCP plan for the meatless snack kits, and a PRP in the USDA plan for the meat-containing snack kits. The monitoring and verification activities could be performed at different frequencies, and corrective actions could be different. Facilities that are audited under any of the various Global Food Safety Initiative auditing schemes will need to justify their decision-making process for the choice of preventive controls, so a well-documented procedure to follow when making these decisions is a necessary component of an HACCP/food safety plan. Facility personnel will need to understand and be able to explain any and all tools that were used to determine the type of preventive control.
Summary
Hazard Analysis for food safety is a complex process and is different for every type of food product and food manufacturing facility. It is easy to get caught up in predetermined schemes and rely on published guidance documents. Those tools, while an excellent starting point, should not be used “straight from the page” but adapted to each unique manufacturing facility scenario. Employing a combination of expert knowledge, use of decision trees and use of risk matrices is the most effective means of arriving at solid Hazard Analysis and preventive control decisions.
Bala Kottapalli, Ph.D., CQE, is Senior Principal Microbiologist, Food Safety & Microbiology at Conagra Brands.
Loralyn H. Ledenbach, M.Sc., is Principal Scientist at The Kraft Heinz Company.
References
1. Almond Board of California. 2007. Considerations for Proprietary Processes for Almond Pasteurization and Treatment, v1.0.
2. Almond Board of California. 2007. Guidelines for Process Validation Using Enterococcus faecium NRRL B-2354, v1.2.
3. Almond Board of California. 2007. Guidelines for Validation of Blanching Processes, v1.0.
4. Almond Board of California. 2007. Guidelines for Validation of Dry Roasting Processes. Guidelines for Validation of Oil Roasting Processes.
5. Grocery Manufacturers Association. 2009. Control of Salmonella in Low-Moisture Foods.
6. Grocery Manufacturers Association. 2010. Industry Handbook for Safe Processing of Nuts.
7. Anderson, DG and LA Lucore. Validating the Reduction of Salmonella and Other Pathogens in Heat Processed Low-Moisture Foods (Alexandria, VA: Alliance for Innovation & Operational Excellence, 2012).
8. Codex Alimentarius Commission. Guideline for the Validation of Food Safety Control Measures (CAC/GL 69-2008) (Rome: Joint FAO/WHO Food Standards Program, 2008).
9. NACMCF. 1998. “Hazard Analysis and Critical Control Point Principles and Application Guidelines.” J Food Prot 61:762–775.
10. NACMCF. 2006. “Requisite Scientific Parameters for Establishing the Equivalence of Alternative Methods of Pasteurization.” J Food Prot 69:1190–1216.
11. NACMCF. 2010. “Parameters for Determining Inoculated Pack/Challenge Study Protocols.” J Food Prot 73:140–202.
12. Scott, VN et al. 2005. “Guidelines for Conducting Listeria monocytogenes Challenge Testing of Foods.” Food Prot Trends 25:818–825.
13. GMA. HACCP — A Systematic Approach to Food Safety: A Comprehensive Manual for Developing and Implementing a Hazard Analysis and Critical Control Point Plan, fifth ed. (2014).
14. Department of Health and Human Services, Public Health Service, Food and Drug Administration Center for Food Safety and Applied Nutrition, Office of Food Safety. Fish and Fishery Products Hazards and Controls Guidance, fourth ed. (2011).
15. Kottapalli, B., A. Cunningham, Y. Huang, E. Akins and S.J. Hermansky. “Survival of Salmonella spp. and L. monocytogenes during Pancake Cooking Process.” Poster presentation at IAFP 2015 Annual Conference, Portland, OR.
16. Kottapalli, B and DW Schaffner. “Modeling the Risk of Salmonellosis Associated with Consumption of Frozen Precooked Pancakes.” Poster presentation at IAFP 2016 Annual Conference, St. Louis, MO.
17. Codex Alimentarius Commission. 2003. “Hazard Analysis and Critical Control Point (HACCP) System and Guidelines for Its Application.” Annex to CAC/RCP 1-1969 (Rev. 4-2003).
18. Codex Alimentarius Commission. CX/FH 17/49/5 Proposed Draft Revision of the General Principles of Food Hygiene (CAC/RCP 1-1969) and its HACCP Annex.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!