Foods Produced by Biotechnology: A Toxicologist’s Perspective on Intended vs. Unintended Changes

“The dose makes the poison.” Whether you attended a presentation or read a scientific article, if a toxicologist was involved, there is a good chance you have heard these words. While seemingly simplistic in its message, this concept, which is foundational to the field of toxicology, has withstood the test of time. It creates a distinction between intended and unintended effects. Depending on the specific industry and product class in question, intended effects are also referred to as pharmacology, efficacy and therapeutic effects, or technical function, while unintended effects can be regarded as off-target effects, adverse effects, or toxicity, based on the severity of the changes. Ultimately, it is crucial to understand the spectrum of these undesired changes for any regulated product to maximize its benefit while minimizing the risk to the end user, a concept that has been dramatically influenced by modern biotechnology.
To illustrate how biotechnology has enabled us to develop more precise and less toxic products, let us examine the changes we have seen in the past 30 years with regard to new drug development. For many years, small-molecule drugs remained the mainstay of therapeutic intervention, while early biologic-type treatments required harvesting and purification from animal sources. With advancements in biotechnology, traditional products like porcine- and bovine-sourced insulin were replaced with recombinant human insulin, which was more effective and resulted in far fewer allergic and autoimmune reactions as well as reduced the potential for exposure to viruses and prions. Similarly, small-molecule drug inhibitors, which tended to have many off-target effects, were steadily replaced with highly specific therapeutic proteins and antibodies, effectively reducing those undesired effects. Some of the most significant improvements were seen in the field of oncology drug development. The historical premise of chemotherapeutic intervention for many years relied on DNA-damaging agents that would target both cancer and normal cells alike, resulting in significant toxicity. Using biotechnology, researchers were able to identify markers specific to cancer cells and develop antibodies and antibody-drug conjugates to target cancer cells, effectively killing them and leaving normal cells unharmed, thereby significantly reducing toxicity. Irrespective of the specific therapeutic class or indication, it is well-accepted that biotechnology has had a very positive impact on the development of more precise and less toxic pharmaceuticals, replacing approaches that were more prone to unintended effects.
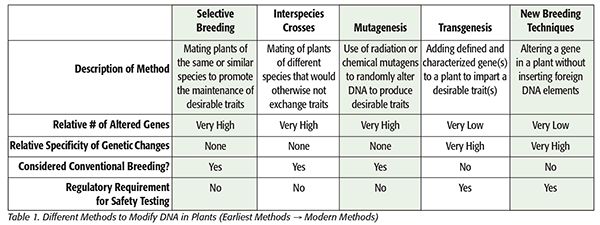 In stark contrast to the welcome advancements in pharmaceutical development, biotechnology’s role in the food industry, despite being introduced around the same time, still faces significant challenges in adoption. The general public’s uncertainties regarding the benefits to the consumer, combined with a complex and inconsistent regulatory environment and a general unwillingness to accept broad scientific consensus on safety, have left the food industry in a constant state of tug-of-war. Our goal in the field of toxicology is to help develop products that deliver on the intended effects while minimizing the unintended effects. When we apply this charge to biotechnology and its use in food production, it is important we critically compare and contrast the alternative methods of food production. Specifically, we need to carefully evaluate conventional breeding methods against modern methods of genetic engineering with regard to intended versus unintended changes to gain a better perspective on the safety of these products (Table 1).
In stark contrast to the welcome advancements in pharmaceutical development, biotechnology’s role in the food industry, despite being introduced around the same time, still faces significant challenges in adoption. The general public’s uncertainties regarding the benefits to the consumer, combined with a complex and inconsistent regulatory environment and a general unwillingness to accept broad scientific consensus on safety, have left the food industry in a constant state of tug-of-war. Our goal in the field of toxicology is to help develop products that deliver on the intended effects while minimizing the unintended effects. When we apply this charge to biotechnology and its use in food production, it is important we critically compare and contrast the alternative methods of food production. Specifically, we need to carefully evaluate conventional breeding methods against modern methods of genetic engineering with regard to intended versus unintended changes to gain a better perspective on the safety of these products (Table 1).
Genetic changes through spontaneous mutations and natural selection have been occurring throughout the evolution of the plant kingdom over millions of years. In addition, selective breeding by humans has been in use since the domestication of edible plants and the development of agriculture over the past several thousand years. Whether by the forces of nature or the hand of man, selective breeding and spontaneous genetic mutations have given rise to the vast diversity we currently see in the plant kingdom, including most of the plant varieties consumed by humans. Interspecies plant breeding is a relatively more recent development. It has been utilized for the past 200 years to develop new plant varieties involving species that would otherwise not be able to exchange traits. Mutagenesis, a comparatively more recent development, is another form of plant breeding, which uses radiation or potent chemical mutagens to introduce random genetic mutations in plants to create new traits. While these three varying techniques are generally regarded as conventional breeding methods, they also share another characteristic in that their respective processes are known to randomly modify a significant number of genes, with little to no specificity toward the desired trait. Most importantly, new plant varieties generated using these conventional breeding methods are not subject to regulatory scrutiny that requires testing to ensure human safety.
Transgenic methods have been used for over 25 years to incorporate well-characterized genes from a foreign (different plant or nonplant species) donor into a plant host that endow it with some desirable trait. This method has given us a number of crop varieties that include traits such as herbicide tolerance and/or pesticidal effects, allowing for the decreased use of crop treatments. The resulting products have been designated by the common moniker of genetically modified organisms or GMOs.
The use of this transgenic method differs from conventional breeding techniques in that the number of genes affected is very low, sometimes limited to a single gene. In addition, the specificity of the genetic changes is very high in that a given inserted gene of interest has a known sequence, coding for a known protein with a known function. More recently, “new breeding techniques” represent the frontier of genetic engineering in plants and is a term used to describe methods like gene editing using CRISPR (clustered regularly interspaced short palindromic repeats) technology as well as RNA interference. These techniques allow for the modification of host plant genes without the incorporation of any foreign DNA, making it a highly precise mutagenesis method that could be limited to a single gene, perhaps even a single base-pair substitution. Unlike plants produced via conventional breeding methods, plant varieties generated by transgenic technology or new breeding techniques are generally subject to extensive regulatory scrutiny, including but not limited to safety testing to show suitability for human and animal consumption as well as safety for the environment.
There are a variety of ways in which the safety of genetically modified plant varieties can be assessed. One of the most powerful tools available to researchers is the Basic Local Alignment Search Tool-Protein, which is maintained by the National Center for Biotechnology Information. This tool allows researchers to evaluate the protein sequences (encoded by inserted genes) added to a plant variety through a genetic engineering technique for similarity to known toxins or allergens. This represents the primary concern from a human health and safety perspective. Combining this with whole-genome sequencing technology and the ability to identify new potential open reading frames, researchers can utilize this bioinformatics approach to predict what proteins will be expressed in a genetically modified plant variety, and whether they present any potential hazard to humans or animals that consume them. An additional approach is to evaluate the digestibility of newly expressed proteins in simulated gastric fluids. The premise behind this approach is that digestion-resistant proteins may represent potential allergenic peptide sequences. While this may not always be the case, the data from such a test could add to the weight of evidence if a particular incorporated gene codes for a protein that bears sequence homology to known toxins or allergens. An in vivo test that has been employed for evaluating the safety of recombinant proteins being expressed in plants is the acute oral toxicity assay in rodents. While this test represents an extreme case of exposure, the goal is to show that the expressed protein does not present a clear health hazard, even if consumed in large quantities. Finally, a basic chemical characterization of the plant variety with regards to nutrient content is warranted to demonstrate that the new plant variety can still deliver on its intended function, which is to provide nourishment.
From a toxicology perspective, the current tools employed to evaluate the safety of genetically modified plant varieties seem appropriate in providing insights on both the intended and unintended changes associated with the respective modification technique utilized. A testament to this demonstration of safety is seen in the high adoption rate of common crops like maize and soy globally, with no reported adverse health effects. However, one cannot help but wonder why there are no regulatory requirements to make similar assessments of safety when it comes to new plant varieties created using mutagenesis, which employs radiation and/or potent chemical mutagens to induce DNA damage, resulting in all manner of genetic modifications including, but not limited to, deletions, mutations, and rearrangements. The number of genetic modifications induced by this conventional breeding method can be astronomically higher than those created via the more modern genetic engineering methods. What is the rationale in essentially turning a blind eye to evaluating the unintended changes associated with this conventional breeding method, when we have the technology readily available to do so? As a toxicologist, I will continue to struggle with this question as there is little to no apparent logic behind it. Anyone involved in evaluating the safety of our food supply should question this apparent scientific and regulatory gap.
In reality, one could also raise questions about the safety associated with time-tested methods like selective breeding and interspecies crosses. Scientific evidence suggests that these methods have the potential to generate a significant number of genetic changes. The prevailing argument for safety of these older techniques, especially selective breeding, is the long history of use. The problem with this argument is that it makes a very significant assumption that genetic changes acquired through the processes of nature are inherently safe. When we consider that the modern diversity we see in plants around the world has evolved from green algae over millions of years, it should become abundantly clear that nature doesn’t always take the side of human safety. After all, nature’s ability to modify genes has given us plants such as hemlock, deadly nightshade, and oleander, which are just a few of the known toxic plants with a proven ability to kill humans. Even if we focus on edible plants, either by the hand of man or by nature, selective breeding has given us varieties that were shown to be highly toxic. In 1967, the Lenape (B5141-6) potato was developed by crossing the Delta Gold potato with Solanum chacoense, a wild potato found in Peru. The resulting Lenape potato, while having a number of desirable traits, including blight and insect resistance, also contained high levels of toxic glycoalkaloids. People who reported consuming this potato developed symptoms similar to food poisoning, which was clearly an unintended consequence of this potato created through this conventional breeding method. In 2003, a very similar case was also reported in New Zealand, involving a zucchini produced via conventional breeding that also contained high levels of a toxin, resulting in numerous reported cases of food poisoning-like symptoms. Taken together, it is clear that plant breeding methods considered to be “conventional” may not always favor the side of safety. Given the high potential for genetic changes, perhaps these “conventional” products should be held to the same scientific and regulatory scrutiny as plants produced via genetic engineering, which, not surprisingly, to date have had no reported adverse health effects.
 Adding to this confusing narrative is the recent ruling by the Court of Justice of the European Union (EU) indicating that products made by new mutagenesis techniques, like CRISPR gene editing, are considered GMOs with regard to regulatory scrutiny. This ruling is counter to the preliminary thinking by the U.S. Department of Agriculture (USDA) that certain plant varieties made via CRISPR gene editing would not be regulated as GMOs as they are at least similar to, if not more precise than, their conventionally bred varieties. The USDA secretary made it clear that this determination was to “allow innovation when there is no risk present.”[1] USDA is not alone in this thinking as a recent Japanese government panel reached the same conclusion, suggesting that gene-edited products should not be held to the same regulatory scrutiny as GMOs in which foreign genes have been inserted. In addition, Australia’s gene technology regulator has also proposed not regulating products of gene editing, similar to the U.S. and Japan. While it is entirely possible that the EU court decision on gene editing technology may become a minority opinion in the future as other regulatory bodies around the globe begin to align with USDA’s view on the matter, one has to wonder why there is such a divergent view on this topic. To better illustrate this discrepancy, let’s examine a potential scenario where we use a “conventional” mutagenesis technique versus gene editing to generate a product with the same desirable trait and consider the unintended changes (Table 2). If we are able to achieve the same desired outcome of a new plant variety with the intended trait from the same starting point, which path should we follow? To a scientist whose focus is on the safety of products, a path that helps clarify the unknowns and minimizes unintended changes would obviously be the preferred route as it presents the safest product for human consumption. However, the EU court opinion would run counter to this assertion as it would favor the older and obviously more imprecise method of mutagenesis simply because of the argument that it has a long history of safe use. Even worse, this decision could have long-term ramifications for biotechnology providers and innovation in the EU.
Adding to this confusing narrative is the recent ruling by the Court of Justice of the European Union (EU) indicating that products made by new mutagenesis techniques, like CRISPR gene editing, are considered GMOs with regard to regulatory scrutiny. This ruling is counter to the preliminary thinking by the U.S. Department of Agriculture (USDA) that certain plant varieties made via CRISPR gene editing would not be regulated as GMOs as they are at least similar to, if not more precise than, their conventionally bred varieties. The USDA secretary made it clear that this determination was to “allow innovation when there is no risk present.”[1] USDA is not alone in this thinking as a recent Japanese government panel reached the same conclusion, suggesting that gene-edited products should not be held to the same regulatory scrutiny as GMOs in which foreign genes have been inserted. In addition, Australia’s gene technology regulator has also proposed not regulating products of gene editing, similar to the U.S. and Japan. While it is entirely possible that the EU court decision on gene editing technology may become a minority opinion in the future as other regulatory bodies around the globe begin to align with USDA’s view on the matter, one has to wonder why there is such a divergent view on this topic. To better illustrate this discrepancy, let’s examine a potential scenario where we use a “conventional” mutagenesis technique versus gene editing to generate a product with the same desirable trait and consider the unintended changes (Table 2). If we are able to achieve the same desired outcome of a new plant variety with the intended trait from the same starting point, which path should we follow? To a scientist whose focus is on the safety of products, a path that helps clarify the unknowns and minimizes unintended changes would obviously be the preferred route as it presents the safest product for human consumption. However, the EU court opinion would run counter to this assertion as it would favor the older and obviously more imprecise method of mutagenesis simply because of the argument that it has a long history of safe use. Even worse, this decision could have long-term ramifications for biotechnology providers and innovation in the EU.
It has often been stated that advances in technology tend to outpace their accompanying regulations. In the case of modern biotechnology, there seems to be more of a divergence in food and agriculture as opposed to other sectors, which have benefited tremendously from its use. The current state of global biotechnology regulations for food is at best inconsistent in its approach and at worst carries a strong nonscientific bias that discourages the use of biotechnology. If we take a scientific approach in evaluating the safety of new plant varieties, we should use all the tools available to best understand the technique used and the modifications it creates. If regulations require us to conduct extensive risk assessments and safety evaluations of transgenic crop varieties, the same standard of safety should be applied for the older, conventional methods of generating new crop varieties. Otherwise, we are effectively going backward by encouraging the use of imprecise plant breeding techniques, which do not always give the intended result (recall the Lenape potato), instead of far more precise genetic engineering techniques. To effectively use modern biotechnology to meet the future challenges of feeding a growing global population, we need a more scientifically robust and risk-based regulatory approach to evaluate the safety of new plant varieties. We can start by simply considering the vast number of unknown genetic modifications in edible plants made using traditional mutagenesis as compared with the surgical precision of gene editing and ask which method should be subject to greater regulatory and safety scrutiny. As a toxicologist responsible for safety evaluations, I think the answer is clear.
Alex Eapen, Ph.D., DABT, is principal scientist – Toxicology & Regulatory, R&D Scientific & Regulatory Affairs at Cargill. The content, remarks, and opinions presented here are those of Dr. Eapen and do not necessarily reflect those of Cargill Inc.
Reference
1. Statement by USDA Secretary Sonny Perdue on plant breeding innovation (March 28, 2018 – USDA Press Release No. 0070.18).
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!







