Sanitation Verification for Allergen Control

This article is a continuation of our survey results from December 2017 of approximately 335 food processors across the U.S. and Canada, as well as 38 other countries, on their concerns about their allergen control and sanitation verification programs. As we mentioned in the last issue,[1] we wanted to hear about their most pressing problems and how they are dealing with the key issues of allergen control and sanitation. This article will focus on sanitation and verification testing.
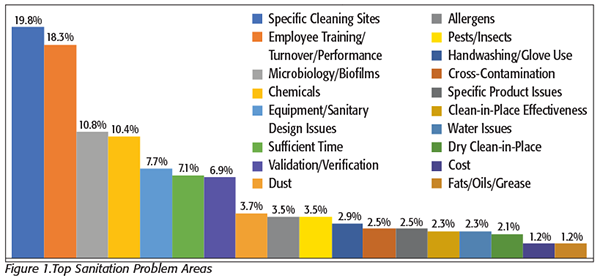 We began the survey with an open-response question about difficult sanitation issues (Figure 1). Taking the top spot was specific cleaning sites.
We began the survey with an open-response question about difficult sanitation issues (Figure 1). Taking the top spot was specific cleaning sites.
The best way to explain this answer is that each processor had a specific difficult cleaning site but few were exactly the same. We found, as expected, the usual answers of floors, walls, drains, belts, overhead piping, and pump housings. But we also found that each processor seemed to have a specific and unique problem area, including chocolate residue on machines (candy manufacturer) and the vegetable ricer machine (frozen food processor), with another processor citing “…cleaning the inevitable burnt-on materials.” One processor’s answer of “one specific tank” that was always a problem seemed to resonate with many of the processors who, when we mentioned that answer, said, “Oh yes, I have one of those too.”
As we’ve discussed before, a sanitation issue that processors always rank high on their list of concerns is employee compliance with cleaning protocols. This survey was no exception, with employee compliance, training, and performance taking second in the list. Again, employee compliance and performance are tied to “sufficient time” as essentially the same issue. “We need more time to complete our work with our current staff” and “We need more people to get our work done in time” were common comments.
Seeking the Right Chemicals
Issues of microbiology and control of pathogens and biofilms continue to come up frequently and ranked third in the survey. The fourth-most mentioned was concerns about chemicals. We have seen an emphasis on chemical issues previously, but they ranked higher in this survey than any in the past. The comments this time seemed to focus more on having the right selection of chemicals to be effective, especially against biofilms and difficult cleaning challenges, and getting the right “mix” of chemicals to be effective than on issues such as disposal, storage, labeling, or related ancillary issues.
Several respondents mentioned that they do not think they get enough help from their chemical provider to determine which is the right product for their specific applications, how to determine the right amount of disinfectant to use, and what is appropriate for their plant, equipment, and product. Some mentioned that the manuals and user guides that come with their chemicals are poor and nonspecific, and several even mentioned that the supplied material safety data sheets were inaccurate and not well written. Another processor commented that they used to have these issues with service from their chemical suppliers, but they were able to solve this by specifically including (and naming) the service and support that they expected in their purchase contracts. This sounds like both a challenge and an opportunity for the chemical companies.
 We asked which chemicals were most used (Figure 2). Companies from the U.S. and Canada reported using quaternary ammonia products (quats) most frequently, with more than 55 percent of the respondents reporting use, with hypochlorites (40.9%) and peracetic acid (23.7%) being the next most frequent. Sanitizers such as alcohols, iodophors, and green chemicals (as well as “other”) were all reported by fewer than 4 percent of the respondents. Outside the U.S. and Canada, most companies reported using hypochlorites most frequently (67.9%), with quats (40.7%) and peracetic acid (28.4%) following. The use of alcohol-containing products was reported three times more frequently than with the U.S./Canada sample, and “other” was also more frequent, although still significantly lower than hypochlorites and quats.
We asked which chemicals were most used (Figure 2). Companies from the U.S. and Canada reported using quaternary ammonia products (quats) most frequently, with more than 55 percent of the respondents reporting use, with hypochlorites (40.9%) and peracetic acid (23.7%) being the next most frequent. Sanitizers such as alcohols, iodophors, and green chemicals (as well as “other”) were all reported by fewer than 4 percent of the respondents. Outside the U.S. and Canada, most companies reported using hypochlorites most frequently (67.9%), with quats (40.7%) and peracetic acid (28.4%) following. The use of alcohol-containing products was reported three times more frequently than with the U.S./Canada sample, and “other” was also more frequent, although still significantly lower than hypochlorites and quats.
The data showed that most of the processors from the U.S. and Canada (52.5%) used more than one type of sanitizer, with 15 percent reporting using three types and 37.5 percent reporting using two types. The results were similar with the international processors, with 40.5 percent using more than one sanitizer type, 12 percent reporting using three, and 29 percent reporting using two. We also asked if they deliberately rotate the sanitizers that they use (for bacterial resistance reasons), and the answers were similar among all processors, with approximately 45 percent saying yes and 55 percent saying that they do not.
Change in ATP Use
 We also asked processors about their use of adenosine triphosphate (ATP) tests in sanitation verification (Figure 3). You may recall that we also reported on the processors’ use of ATP as part of their allergen control program in the last issue, but these additional questions were directed specifically at their use in sanitation verification (although it is clear that these two applications are interrelated).
We also asked processors about their use of adenosine triphosphate (ATP) tests in sanitation verification (Figure 3). You may recall that we also reported on the processors’ use of ATP as part of their allergen control program in the last issue, but these additional questions were directed specifically at their use in sanitation verification (although it is clear that these two applications are interrelated).
For processors in the U.S. and Canada, 60.5 percent reported that they use ATP, and 39.5 percent said they are not currently using ATP (although several companies said they “were considering” starting a program using ATP). These companies used an average of 64 tests per week (with a low amount of one test per week, a high of 1,000 tests/week, and a median test number of approximately 20–25 tests per week).
We also had 84 international companies report ATP use; of these, 45.2 percent reported using ATP, whereas 54.8 percent said that they did not. Of those using ATP, the companies used an average of 27 tests per week (with a low of one test per week, a high of 250 tests per week, and a median test number of approximately 10 tests per week).
These findings may indicate that processors outside the U.S. and Canada may have an opportunity to improve their sanitation programs by adopting ATP for verification; the rate and volume of use may indicate that there may be growth opportunities for the diagnostic companies that sell ATP tests to expand to new global markets.
We were somewhat surprised by the number of companies that reported that they are now using ATP swabs only “sometimes” or “occasionally.” These companies, rather than saying that they are using ATP swabs on a daily basis to verify routine sanitation work, are reporting that they have changed their policies and are using ATP tests at less frequent intervals, typically monthly or quarterly.
We explored this further in the interviews we conducted. One company mentioned that they had been conducting ATP tests on a regular basis for many years and they now felt that they had a very good baseline and understanding of trends they will see. This, they said, gave them no compelling reason to continue testing daily just to collect more data. They said that they plan to now test quarterly, or in response to specific situations where they felt the testing would be informative. Another quality assurance manager told us that they were stretched for manpower and this constrained their ability to collect daily samples. They had reverted to a schedule of collecting “a few hundred” ATP samples on an approximately quarterly basis. They would also continue to use the tests in training to help new employees with feedback on their work. Others echoed this reporting that they will continue to use ATP for handwashing training and “spot inspections” but no longer daily.
This will be an interesting trend to watch. There were not yet enough companies reducing their use to make a significant impact on the size of the market. But should this trend continue, it will certainly constrain the growth in the market unless the diagnostic companies can continue to demonstrate the value of daily use.
In our next Food Safety Insights, we will be looking again at pathogen testing but this time with a focus on testing for Listeria. Processors are reevaluating their testing programs—especially their environmental monitoring programs—in light of many factors such as recent recalls and outbreaks of Listeria, federal regulations and guidance, concerns about biofilms and harborage organisms, and the threat of U.S. Food and Drug Administration “swab-a-thons.” But regardless of the specific reason, testing for Listeria is growing faster than perhaps any other microbiological testing category. We will find out more and report to you in the next issue!
Bob Ferguson is the managing director of Strategic Consulting Inc. and can be reached at foodsafetyinsights@gmail.com.
Reference
1. www.food-safety.com/magazine-archive1/februarymarch-2018/testing-and-sanitation-for-allergen-control/.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!





.webp?t=1721343192)

