The Need for Ongoing Confirmation of Food Safety Programs Efficacy
Food businesses that do not confirm the efficacy of what they do to ensure the safety and quality of food are inevitably left at the mercy of repeating failures

Image credit: passorn santiwiriyanon/E+ via Getty Images
The current industry landscape does not lend itself to the confirmation of food safety systems efficacy. Food operators may not even know what this means. Ignoring the confirmation of systems efficacy leaves us with only the prospect of remaining in the rut of incessant food safety failures.
In the general industry discourse, one mostly hears "dream world" proposals, unverified claims, and misleading conclusions. Dreamers loudly describe their exploits as they rock wooden horses and expect to reach some imaginary food safety "destination" (Figure 1).
This article suggests that better is possible. It reaffirms the necessity of ongoing confirmation of the efficacy of food safety proposals, pursuits, and programs. Food businesses that do not confirm the efficacy of what they do to ensure the safety and quality of food are inevitably left at the mercy of repeating failures that are preventable, dragging the entire industry along on this regrettable path.

History of Diminishing Commitment to Efficacy Confirmation
Weinroth, Belk, and Belk exposed this endemic flaw in modern-day food safety management systems.1 These authors did not mention, even implicitly, that efforts in the industry are not yielding desirable results. The authors are not to blame for having no results to report. Like the rest of the industry, they are engulfed in prevarication, obfuscation, and paltering ('POP') culture.
Artisan and scientific inventions historically incorporated the confirmation of repeatable and reproducible efficacy as part of the scientific method that proceeds from hypothesis to theory to law. For example, Nicolas Appert, a French chef, applied the scientific method and invented the process of preserving food by enclosing it in hermetically sealed containers (the canning process) with proven efficacy.2 In the 1860s, Louis Pasteur demonstrated that abnormal fermentation of wine and beer could be prevented by heating the beverages to approximately 57 °C (135 °F) for a few minutes, yielding the pasteurization process with proven efficacy.3
The hyperbaric (high-pressure) food processing, food irradiation, and other food treatment methods with proven food safety efficacy emerged in later years. Sadly, the industry appears to have abandoned the scientific methods of confirming the efficacy of food operation and food safety control systems.
The Survey of Food Laws and Regulations discusses food regulations starting with old-era trade guilds. Modern regulations have since evolved with a noticeable decline in the confirmation of efficacy, due to (or resulting from) a general loss of focus on the mandate of regulators around the world.4
New-era industry initiatives and assessment schemes have also followed the pattern of focusing only on compliance and failing to confirm the efficacy of implemented systems. The resulting 'POP' culture is most vivid with the commercialized third-party audit schemes.
Conventional Food Safety and Quality Controls, Monitoring, and Measurement
The food industry employs several methods for ensuring that expected "standards" are maintained, although not always with a primary focus on effectiveness.
A food enterprise must meet regulatory and customer requirements. Meeting regulations involves implementing documented food safety plans, including the 12 steps of HACCP. Customers also require their suppliers to implement food safety management programs that align with compliance-based systems like Global Food Safety Initiative (GFSI) "standards" (more correctly, schemes). Most operation effectiveness monitoring systems are based on these limited and limiting scheme requirements and regulations. Food companies may feel constrained to the requirements prescribed by the schemes instead of taking the initiative to implement programs with the necessary confirmation of efficacy.
Since commercialized schemes and regulations attempt to cover all product categories across a number of sectors and operation stages, they can only be generic. The prescribed activities tend to be frivolous, and they often deviate from the ultimate goal of ensuring safe food. For example, they do not quantitatively track how safe food has become. They focus more on generic requirements that may or may not be applicable to the operations audited or inspected.
Imagine a warehouse dealing with an active rodent infestation. Instead of addressing the root cause of the problem with a focus on the efficacy of controls, the quality technician mostly focuses on ensuring that placards are precisely positioned above each pest control trap to avoid audit nonconformance. This focus on minor compliance details illustrates the potential pitfalls for facilities that prioritize strict adherence to the schemes over actual efficacy. This warehouse may need to deviate from irrelevant scheme prescriptions if it wishes to ensure the efficacy of the pest control program to achieve sustainable and long-term effectiveness.
Efficacy and Effectiveness
"Efficacy" refers to the capacity (or ability) of food safety and quality assurance (FSQA) programs, under controlled conditions, to achieve their intended outcomes of preventing foodborne illnesses; minimizing product recalls; ensuring regulatory compliance; and maintaining consumer safety, satisfaction, and trust. Efficacy, in this context, means that the implemented measures have the power to successfully mitigate risks and to deliver safe and high-quality food products consistently.
The "efficacy" and "effectiveness" of programs are related, but with specific distinctions. Efficacy pertains to accomplishing the desirable objectives of the programs, while effectiveness pertains to measuring how well the programs work in real-world, practical conditions, when applied with consideration to factors such as variability in implementation, human error, and environmental influences. Briefly stated: Efficacy ensures, while effectiveness measures the actual success in real-world situations.
Figure 2 shows the conventional control elements and sub-elements of an FSQA system. It lists the effectiveness measurement activities. This graphic does not display an all-inclusive list, but rather serves as a general overview of the monitoring and measurements of system effectiveness. The sub-elements can be further broken down into customized tasks and methods according to the nature and unique needs of the operation.
The conventional FSQA effectiveness measurements pertaining to each of the sub-elements include:
- Visual observations
- Validation activities and testing
- Verification activities
- Customer/consumer feedback.

The activities shown in Figure 1 are typical requirements for facilities seeking certification under GFSI food safety management schemes. HACCP and Preventive Control plans essentially combine as the Food Safety Plan. Food operations that have good technical guidance concurrently implement these concepts and requirements.
To ensure a robust system with efficacy, a food business needs to conduct relevant hazard and risk assessments to determine which programs are required. A food business also needs to conduct evaluations of implemented program outcomes to assess the levels of success achieved. Implementing futile programs creates waste and undermines the ability to achieve the desirable ultimate goals.
S.W.O.T Analysis of Current Food Business Food Safety Performance
The below analysis highlights areas of strength to leverage, weaknesses to address, opportunities to pursue, and threats to mitigate. The following lists are only examples and not an exhaustive listing.
Strengths
- Availability of qualified, competent, experienced, and committed personnel and professionals
- Existence of proven concepts and management strategies
- Internal monitoring, auditing, and training programs
- Consumer feedback mechanisms
- Resource availability from regulators, research bodies, good industry associations, and international organizations like the World Health Organization (WHO)
- Availability of effective regulatory oversight.
Weaknesses
- Resource constraints
- Complex supply chain
- Complacency, complicity, or confusion of standards/schemes
- Unorganized solutions applied to sporadic problems
- Slowness in relevant technological adoption
- Absence of adequate support from regulators.
Opportunities
- Technological advancements
- Collaboration with industry partners
- Collaborating with regulators
- Management concepts and strategies available for adoption.
Threats
- Environmental and climate-related challenges
- Emerging foodborne pathogens and other hazards
- Supply chain disruptions
- Foodborne illness outbreaks and litigation.
Weakness in Confirming the Efficacy of Implemented Food Safety Programs
Conventional proposals, programs, practices, and monitoring schemes are mostly based on theoretical assumptions, subjective projections, and unverified conclusions and imaginations about "food safety outcomes." Claims like "the development of HACCP has led to improvement in food safety outcomes" are made without providing substantiating evidence.
The HACCP concept, in fact, has the inherent capacity (efficacy) to deliver safe food, but not when it is badly implemented (Figure 3). Repeating foodborne illness outbreaks and recalls show a disappointing record of ensuring the safety of food worldwide. The WHO provides indisputable evidence of global food system failures in its reports by estimating the global burden of foodborne diseases.5

What Must Follow?
The industry needs to speak the language that radically and positively alters the trajectory of the 'POP' culture. An excessive focus on meeting the dictates of schemes based on superficial and easily fabricated compliance must be replaced by systems that are designed to continuously confirm the efficacy of proposals, plans, and programs.
To effect real change, determined actions must be met with moral and ethical value propositions at every level. The "selling" of fake solutions that cannot be substantiated as providing value, efficacy, effectiveness, and efficiency must stop. Considering the much-needed culture change, and according to their respective areas of focus and needs, it is imperative that food businesses align with the following goals:
- Get out of the third-party certification tunnel (Figure 4)
- Seek and learn from the right coaches, not the opportunists who sell only temporary solutions
- Act beyond merely talking about issues
- Confirm that desirable safer food outcomes are consistently achieved according to desirable goals and mission objectives.
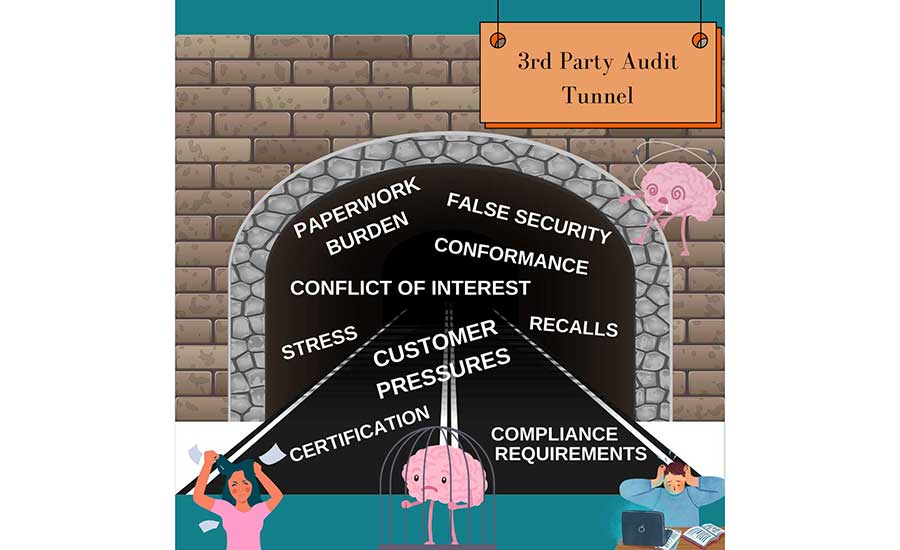
Recommendations
With a brief review of historical developments, this article laments the endemic failure of the food industry to confirm the efficacy of its FSQA systems. It defines efficacy as the ability of food system controls to achieve desirable outcomes like preventing foodborne illnesses and recalls. Past discoveries like canning and pasteurization followed scientific methods. These discoveries have shown indisputable efficacy in ensuring the safety of food. Modern-day systems need to do the same by design and in their deployment.
Food businesses need to move beyond the third-party audit systems that are driven by distrust and sustained mostly by assumptive conclusions. This article advocates a radical abandonment of superficial compliance assessments in favor of systems that ensure real-world confirmation of efficacy. It recommends genuine and reliable evaluation of safer food outcomes through systems that follow the scientific method.
References
- Weinroth, M.D., A.D. Belk, and K.E. Belk. "History, development, and current status of food safety systems worldwide." Animal Frontiers 8, no. 4 (August 2018): 9–15. https://doi.org/10.1093/af/vfy016.
- Encyclopedia.com. "Nicolas Appert." May 21, 2018. https://www.encyclopedia.com/people/food-and-drink/food-and-cooking-biographies/nicolas-appert.
- Encyclopedia Britannica. "Pasteurization, heat-treatment process." 2024. https://www.britannica.com/technology/pasteurization.
- Amiri, F. Food Safety & Quality Assurance: A Survey of Food Laws & Regulations. Seattle, Washington: Kindle Books. 2023. https://www.amazon.com/dp/B0C2WDHFWG.
- World Health Organization. "WHO Estimates of the Global Burden of Foodborne Diseases." 2015. https://iris.who.int/bitstream/handle/10665/199350/9789241565165_eng.pdf.
Felix Amiri is the Technical Director at Amiri Food Industry Support (AFIS) Services. He also initiated the International Food Industry Think Tank meetings. Felix teaches food safety and quality assurance courses, including International Food Law and Regulations, at Conestoga College. He is also the lead instructor at the SSQA Academy and the author of the books Food Protection Diaries, Efficacy Versus Compliance, Commitment Plans for National & International Regulations, and Food Safety & Quality Assurance: A Survey of Food Laws & Regulations.
Cori Muse is a Food Safety and Regulatory Consultant and the Owner of Muse Food Safety Solutions LLC. She is also an independent GFSI Auditor, an alumni of PepsiCo/Frito-Lay, and has an extensive background working in the food industry over 14 years. She can be reached at solutions@foodsafetymuse.com.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!







