FDA
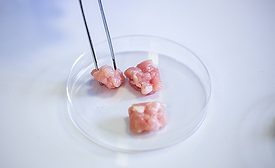
What Was Not Learned from the FDA Investigation of Salmonella on Cantaloupe in 2022?
With another cantaloupe outbreak occurring in 2023, change needs to happen, or there will likely be another outbreak in 2024
December 12, 2023
Never miss the latest news and trends driving the food safety industry
eNewsletter | Website | eMagazine
JOIN TODAY!Copyright ©2024. All Rights Reserved BNP Media.
Design, CMS, Hosting & Web Development :: ePublishing